저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게
l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다:
l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다.
l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다.
저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다.
Disclaimer
저작자표시. 귀하는 원저작자를 표시하여야 합니다.
비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다.
변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Melatoninattenuates cisplatin-inducednephrotoxicitythrough dual suppressionof apoptosisandnecroptosis
김 종 우
2 0 19 년
8 월
석 사 학 위 논 문
Melatonin attenuates cisplatin-induced
계 명 대 학 교 대 학 원
의 학 과
김 종 우
지 도 교 수 박 재 형
2 0 1 9 년 8 월
nephrotoxicity through dual suppression
of apoptosis and necroptosis
nephrotoxicity through dual suppression
김 종 우
Melatonin attenuates cisplatin-induced
지 도 교 수 박 재 형
이 논 문 을 석 사 학 위 논 문 으 로 제 출 함
2 0 1 9 년 8 월
계 명 대 학 교 대 학 원
의 학 과
of apoptosis and necroptosis
김종우의 석사학위 논문을 인준함
주 심 배 재 훈
부 심 박 재 형
부 심 송 대 규
계 명 대 학 교 대 학 원
Table of Contents
1. Introduction ··· 1
2. Materials and Methods ··· 3
3. Results ··· 7
4. Discussion ··· 17
5. Summary ··· 20
References ··· 21
Abstract ··· 26
국문초록 ··· 28
List of Figures
Figure 1. Effect of melatonin on renal function in cisplatin-treated mice ··· 10
Figure 2. Effect of melatonin on tubular damage in cisplatin-treated mice ··· 11
Figure 3. Effects of melatonin on expression of tubular injury markers in cisplatin-treated mice ··· 12
Figure 4. Effect of melatonin on cisplatin-induced tubular apoptosis
··· 13
Figure 5. Effect of melatonin on cisplatin-induced necroptosis in the kidneys ··· 14
Figure 6. Effect of melatonin on cisplatin-induced inflammation in the kidneys ··· 15
Figure 7. Effects of melatonin on cisplatin-induced apoptosis and necroptosis in cultured mouse renal tubular epithelial cells
··· 16
1. Introduction
Cisplatin is a chemotherapeutic drug that has been applied worldwide to deal with many types of cancer, including head, neck, lung, breast, testis and ovary cancers. However, the clinical use of cisplatin is often restricted due to its severe side effects such as nephrotoxicity, neurotoxicity, and ototoxicity (1,2). Especially, the nephrotoxicity of cisplatin is dose-dependent and thereby restricts the possible amount of administering drug. Even though some procedures including hydration management are applied, there is no specific pharmacotherapy for cisplatin-induced nephrotoxicity.
The mechanism of cisplatin-induced nephrotoxicity is complex and includes multiple cellular and molecular pathways (3). Among them, renal tubular cell apoptosis has been thought as a major contributor to the progress of cisplatin-induced damage. Additionally, accumulating evidence indicates that necroptosis, a type of regulated necrotic cell death, also plays an important role in the mechanism of cisplatin-induced nephrotoxicity (4-7). Thus, renal tubular cell death, including apoptosis and necroptosis, may represent an upcoming target for the prevention and therapy of cisplatin-induced injury. Indeed, a recent study reported a synergistic effect of apoptosis and necroptosis inhibitors in cisplatin-induced nephrotoxicity (8).
Melatonin is a hormone produced by the pineal gland and plays a key role in the control of the sleep-wake cycle (9). Together with the role of melatonin in the control of circadian rhythm, past studies have showed that melatonin plays a protective role against cisplatin-induced
nephrotoxicity (10-12). The advantageous effects of melatonin were related with its anti-oxidant and anti-inflammatory properties. Recent studies also showed that melatonin has an inhibitory effect on apoptosis and necroptosis in various disease models such as cardiac ischemia-reperfusion injury (13-15) and liver fibrosis (16,17). However, the effect of melatonin on apoptosis and necroptosis in cisplatin-induced nephrotoxicity hasn’t yet been investigated.
This study showed that administration of melatonin inhibits apoptosis and necroptosis in renal tubules of cisplatin-treated mice, resulting in amelioration of renal dysfunction and histological abnormalities.
Cisplatin-induced apoptosis and necroptosis were also effectively suppressed by melatonin in mouse renal tubular epithelial cells. These results reveal a novel mechanism underlying the safeguarding effect of melatonin against cisplatin-induced kidney injury.
2. Materials and Methods
2.1. Animals:
C57BL/6 male mice (Hanasangsa, Busan, South Korea) were sorted into three groups: control (n = 10), cisplatin alone (n = 10), and cisplatin plus melatonin (n = 10). For cisplatin treatment, mice were injected 15 mg/kg cisplatin (Sigma-Aldrich, St. Louis, MO, USA) intraperitoneally.
Control mice were intraperitoneally injected with the same volume of 0.9% normal saline. Mice were given a intraperitoneal treatment of 20 mg/kg melatonin (Cayman Chemical, Ann Arbor, MI, USA). The amounts of cisplatin (18,19) and melatonin (20,21) were decided on past studies. Blood and kidney tissue samples were collected 3 days after cisplatin injection. All animal experiments were approved by the Keimyung University Institutional Ethics Committee (KM-2017-40R1).
2.2. Cell Culture and Treatments:
Mouse renal tubular epithelial (TCMK-1) cells were bought from Korean Cell Line Bank (Seoul, South Korea). The cells were cultured in Dulbecco’s Modified Eagle Medium (Welgene, Gyeongsan, South Korea) supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin solution and maintained in a moisturized incubator at 37 ℃ under 5% CO₂ and 95% air. TCMK-1 cells were pretreated with 1 mM melatonin for 1 h and after that treated with 2 μ g/mL cisplatin for 24 h.
2.3. Renal Function Test:
Along with the manufacturer’s instructions, blood urea nitrogen (BUN) and creatinine levels were detected using the QuantiChrom Urea and Creatinine Assay Kits (Bioassay Systems, Hayward, CA, USA).
2.4. Histological Analysis:
Renal tissues were placed in 4% paraformaldehyde. Paraffin-embedded tissue blocks were cut into 10 μm sections. Hematoxylin and eosin (H&E) and periodic acid-schiff (PAS) stains were used. Histological analysis was performed using the confocal microscope (NIKON, Tokyo, Japan). PAS-stained tissues were used to detect the tubular damage in randomly selected fields and scored as previously described: 0, normal;
1, 1―10%; 2, 11―25%; 3, 26―45%; 4, 46―75%; and 5, 76―100% (22).
Primary antibodies against neutrophil gelatinase-associated lipocalin (NGAL; Abcam, Cambridge, MA, USA) or kidney injury molecule-1 (Kim-1; Sigma-Aldrich) were treated for 24 h at 4 °C.
2.5. Apoptotic Cell Death Analysis:
Apoptotic cell death was measured using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (Roche Diagnostics, Indianapolis, IN, USA). Deparaffinized tissues were incubated in the TUNEL reaction mixture for 1 h at 37 °C. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The number of TUNEL-positive cells was counted using the confocal microscope.
2.6. Lactate Dehydrogenase (LDH) Release Assay:
Cell death was quantified by measuring the activity of LDH which is released into the cultural medium, because of the disruption of membrane integrity. The results were evaluated by using a commercial kit (Sigma-Aldrich) along with the manufacturer’s instruction. Activity of LDH was measured at 490 nm with a spectrophotometric microplate reader.
2.7. Western Blot Analysis:
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used for the protein resolving. The resolved proteins were transferred to polyvinylidene fluoride membrane. The membrane was incubated with the following primary antibodies: anti-poly ADP-ribose polymerase 1 (anti-PARP-1; Abcam), anti-cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA), anti-receptor-interacting serine/threonine-protein kinase 1 (anti-RIPK1; Cell Signaling Technology), anti-RIPK3 (Cell Signaling Technology), anti-phospho-nuclear factor kappa-light-chain-enhancer of activated B p65 (anti-phospho-NKκB p65; Sigma-Aldrich), anti-Kim-1 (Sigma-Aldrich) and anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH; Cell Signaling Technology). Relative expression level of proteins was analyzed using NIH ImageJ software.
2.8. Quantitative Polymerase Chain Reaction (PCR):
Using TRIzol Reagent (Sigma-Aldrich), total RNA was isolated from
the tissues and then reverse transcribed into cDNA using 5 x First Strand Buffer (Invitrogen, Carlsbad, CA, USA), dNTP Mix (Promega, Madison, WI, USA), OligodT (Macrogen, Seoul, South Korea), Reverse Transcriptase (Invitrogen), and Ribonuclease Inhibitor (Invitrogen). Using the Real-Time PCR 7500 system (Applied Biosystems, Foster City, CA, USA), quantitative PCR was conducted. The specific genes’ mRNA levels were normalized with ribosomal protein L32. The specific primers for tumor necrosis factor-alpha (TNF-α) were 5´-CCC TCA CAC TCA GAT CAT CTT CT-3´ (forward) and 5´-GCT ACG ACG TGG GCT ACA G–3´ (reverse). The specific primers for interleukin-6 (IL-6) were 5´-TAG TCC TTC CTA CCC CAA TTT CC–3´ (forward) and 5´-TTG GTC CTT AGC CAC TCC TTC–3´ (reverse). The specific primers for monocyte chemoattractant protein-1 (MCP-1) were 5´-TTA AAA ACC TGG ATC GGA ACC AA–3´ (forward) and 5´-GCA TTA GCT TCA GAT TTA CGG GT–3´ (reverse). The specific primers for L32 were 5´-ACA TTT GCC CTG AAT GTG GT–3´ (forward) and 5´-ATC CTC TTG CCC TGA TCC TT–3´ (reverse).
2.9. Statistics:
The results are expressed as the mean ± standard error of the mean (SEM). SPSS version 20.0 (SPSS, Chicago, IL, USA) was applied for statistical analysis. Comparisons between two groups were conducted using a Student's two-tailed t-test. A value of p ˂ 0.05 was deemed statistically significant.
3. Results
3.1. Melatonin Ameliorated Cisplatin-Induced Nephrotoxicity:
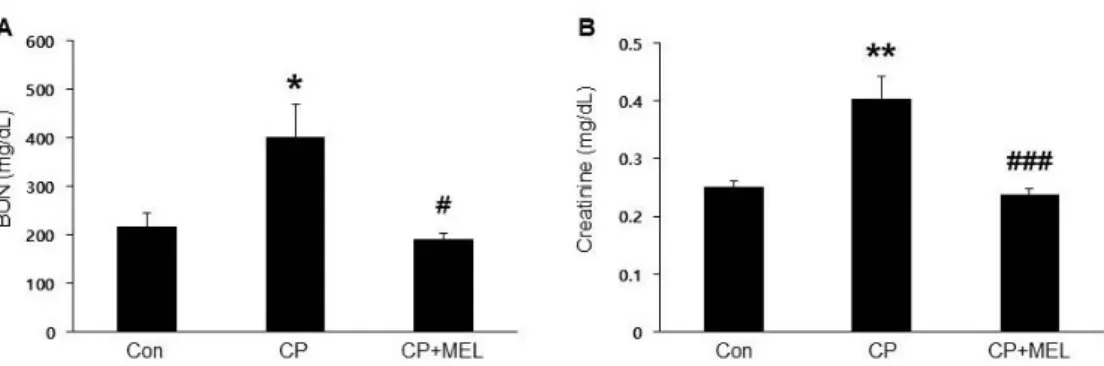
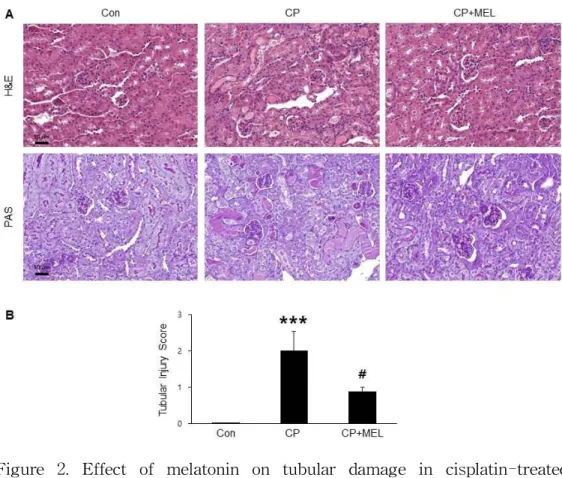
To induce acute kidney injury, mice were treated with cisplatin intraperitoneally. Administration of melatonin significantly attenuated a serious worsening of kidney function, as determined by reduced serum BUN (Figure 1A) and creatinine (Figure 1B) levels. H&E and PAS staining also showed that melatonin treatment significantly ameliorated cisplatin-induced histological changes like as tubular cast formation, tubular dilatation and tubular cell death (Figure 2A&B).
To further evaluate the effect of melatonin in cisplatin-induced tubular damage, the expression levels of tubular injury markers, including NGAL and Kim-1, were examined. In the cisplatin-treated mice, immunohistochemical staining revealed that expression levels of NGAL and Kim-1 were increased in the damaged tubules. These changes were significantly reduced by melatonin (Figure 3A&B). Consistently, melatonin treatment also significantly dropped the expression of Kim-1 (Figure 3C&D).
3.2. Melatonin Attenuated Cisplatin-Induced Tubular Epithelial Cell Apoptosis:
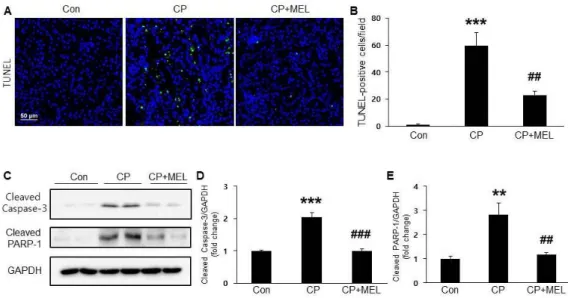
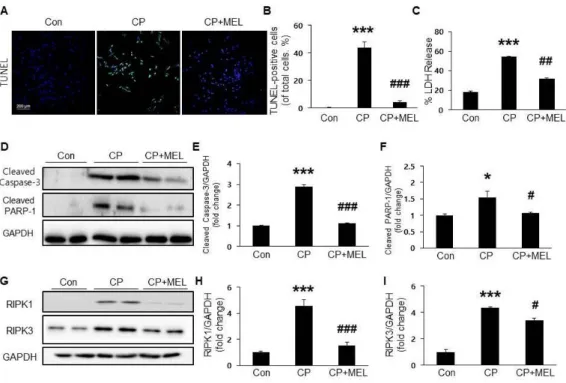
To investigate the mechanisms underlying the safeguarding effects of melatonin against cisplatin-induced nephrotoxicity, TUNEL staining of kidney sections was conducted. Administration of melatonin remarkably
dropped the increased number of apototic cells in the renal tubules (Figure 4A&B). Moreover, melatonin treatment also significantly dropped the ciplatin-induced elevation of cleaved caspase-3 and cleaved PARP-1 expressions (Figure 4C-E).
3.3. Melatonin Attenuated Cisplatin-Induced Tubular Epithelial Cell Necroptosis:
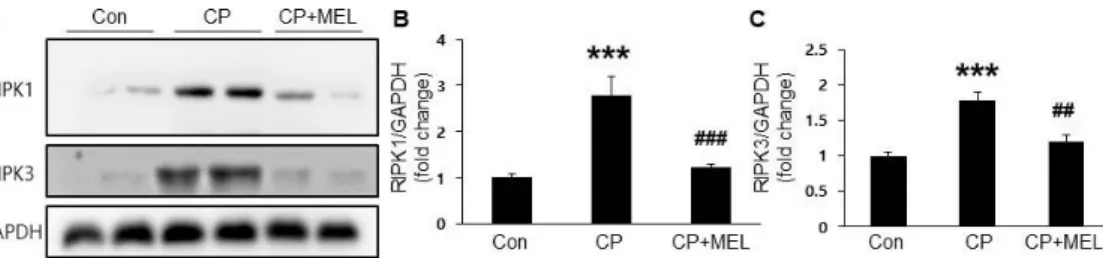
To investigate the effect of melatonin on cisplatin-induced necroptosis, protein expression of RIPK1 and RIPK3 in the kidneys was examined.
Cisplatin treatment markedly increased their protein levels in the kidneys and these changes were significantly attenuated by melatonin (Figure 5A-C), suggesting that melatonin inhibits cisplatin-induced necroptosis.
3.4. Melatonin Attenuated Inflammatory Responses in the Kidneys of Cisplatin-Treated Mice:
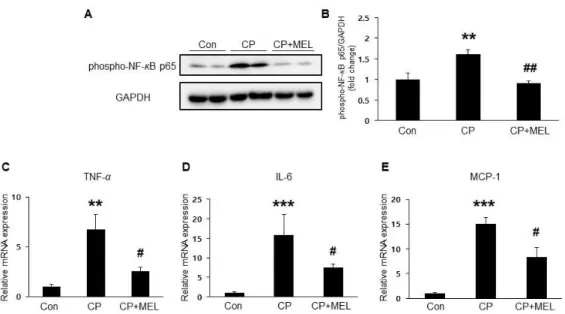
Plasma membrane rupture in necroptosis results in release of intracellular components that stimulate immune system and inflammation (23). Given that cisplatin induces NF-κB activation and subsequently promotes expression of pro-inflammatory mediators, the effect of melatonin on NF-κB phosphorylation in the kidneys was examined.
Administration of melatonin significantly reduced protein level of phospho-NF-κB (Figure 6A&B), in the kidneys of cisplatin-treated mice.
Additionally, pro-inflammatory cytokines’ mRNA expression including TNF-α, IL-6, and MCP-1 were also significantly reduced by melatonin (Figure 6C-E).
3.5. Melatonin Attenuated Cisplatin-Induced Apoptosis and Necroptosis:
Next experiment was whether melatonin could also inhibit cisplatin-induced apoptosis and necroptosis in cultured TCMK-1 cells.
The result was that pretreatment with melatonin dropped the increased number of TUNEL-positive cells after cisplatin treatment (Figure 7A&B). LDH release assay also showed that melatonin significantly reversed cisplatin-induced LDH release (Figure 7C). In addition, elevated cleaved caspase-3 and cleaved PARP-1 in cisplatin-treated cells were significantly decreased by melatonin pretreatment (Figure 7D-F).
Pretreatment with melatonin also significantly reduced the elevated RIPK1 and RIPK3 in cisplatin-treated cells (Figure 7G-I).
Figure 1. Effect of melatonin on renal function in cisplatin-treated mice.
Mice were treated with 20 mg/kg melatonin intraperitoneally.
(A) Serum blood urea nitrogen (BUN) levels. (B) Serum creatinine levels. Con: control; CP: cisplatin; CP+MEL:
cisplatin plus melatonin. n = 10 per group. The values represent the mean ± SEM. *p < 0.05, **p < 0.01 vs. Con. #p
< 0.05, ###p < 0.001 vs. CP.
Figure 2. Effect of melatonin on tubular damage in cisplatin-treated mice. (A) Renal hematoxylin and eosin (H&E) and periodic acid-schiff (PAS) stainings. Scale bar, 50 μm. (B) Tubular injury score. Con: control; CP: cisplatin; CP+MEL: cisplatin plus melatonin. n = 10 per group. The values represent the mean ± SEM. ***p < 0.001 vs. Con. #p < 0.05 vs. CP.
Figure 3. Effects of melatonin on expression of tubular injury markers in cisplatin-treated mice. Renal immunohistochemical staining using anti-neutrophil gelatinase-associated lipocalin (anti-NGAL) antibody (A) and anti-kidney injury molecule-1 (anti-Kim-1) antibody (B). Scale bar, 25 μm. (C) Renal expression level of Kim-1 protein. (D) Relative expressions of Kim-1. Con: control; CP: cisplatin; CP+MEL: cisplatin plus melatonin. n = 10 per group. The values represent the mean
± SEM. **p < 0.01 vs. Con. ##p < 0.01 vs. CP.
Figure 4. Effect of melatonin on cisplatin-induced tubular apoptosis. (A) Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining on kidney sections.
Scale bar, 50 μm. (B) Number of TUNEL-positive cells. (C) Renal expression levels of cleaved caspase-3 and cleaved poly ADP-ribose polymerase 1 (PARP-1). Relative expressions of cleaved caspase-3 (D) and cleaved PARP-1 (E). Con: control;
CP: cisplatin; CP+MEL: cisplatin plus melatonin. n = 10 per group. The values represent the mean ± SEM. **p < 0.01, ***p
< 0.001 vs. Con. ##p < 0.01, ###p < 0.001 vs. CP.
Figure 5. Effect of melatonin on cisplatin-induced necroptosis in the kidneys. (A) Renal expression levels of receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and RIPK3. Relative expressions of RIPK1 (B) and RIPK3 (C). Con: control; CP:
cisplatin; CP+MEL: cisplatin plus melatonin. n = 10 per group.
The values represent the mean ± SEM. ***p < 0.001 vs. Con.
##p < 0.01, ###p < 0.001 vs. CP.
Figure 6. Effect of melatonin on cisplatin-induced inflammation in the kidneys. (A) Renal expression level of phospho-nuclear factor kappa-light-chain-enhancer of activated B (phospho-NF-κB) p65. (B) Relative expressions of phospho-NF-κB p65. Relative mRNA expressions of renal tumor necrosis factor-alpha (TNF- α) (C), interleukin-6 (IL-6) (D), and monocyte chemoattractant protein-1 (MCP-1) (E). Con: control; CP: cisplatin; CP+MEL:
cisplatin plus melatonin. n = 10 per group. The values represent the mean ± SEM. **p < 0.01, ***p < 0.001 vs. Con. #p < 0.05, ##p
< 0.01 vs. CP.
Figure 7. Effects of melatonin on cisplatin-induced apoptosis and necroptosis in cultured mouse renal tubular epithelial cells. The cells were pretreated with melatonin (1 mM) for 1 h and after that treated with cisplatin (2 μg/mL) for 24 h. (A) Renal TUNEL stainings. Scale bar, 200 μm. (B) TUNEL-positive cells. (C) Lactate dehydrogenase (LDH) release assay. (D) Cellular expression levels of cleaved caspase-3 and cleaved PARP-1. Relative expressions of cleaved caspase-3 (E) and cleaved PARP-1 (F). (G) Cellular expression levels of RIPK1 and RIPK3. Relative expressions of RIPK1 (H) and RIPK3 (I).
Con: control; CP: cisplatin; CP+MEL: cisplatin plus melatonin. n
= 10 per group. The values represent the mean ± SEM. *p <
0.05, ***p < 0.001 vs. Con. #p < 0.05, ##p < 0.01, ###p < 0.001 vs.
CP.
4. Discussion
This study showed that melatonin treatment significantly inhibited apoptosis and necroptosis in renal tubules of cisplatin-treated mice.
These effects may contribute to amelioration of cisplatin-induced renal dysfunction and histological abnormalities. Consistent with in vivo data, cisplatin-induced apoptosis and necroptosis in TCMK-1 cells were also effectively suppressed by melatonin. These findings provide a novel mechanism underlying the safeguarding effect of melatonin against cisplatin-induced nephrotoxicity.
Nephrotoxicity is the most important dose-restricting complication of cisplatin-based chemotherapy. Thus, development of effective pharmacotherapy for cisplatin-induced kidney injury can be a strong clinical impact. In this study, administration of melatonin significantly ameliorates renal dysfunction in cisplatin-treated mice. Structural abnormalities were also significantly attenuated by melatonin. Because cisplatin has been known to induce severe tubular injuries in the kidneys (24,25), the effects of melatonin on expression of tubular injury markers such as NGAL and Kim-1 should be examined. In the experiment, increased expression of NGAL and Kim-1 were significantly dropped by melatonin, suggesting the safeguarding effect of melatonin against cisplatin-induced tubular injury. These results indicate that melatonin attenuates cisplatin-induced renal dysfunction and histological abnormalities in mice.
Tubular apoptosis is thought as a key process in the pathogenesis of
cisplatin-induced nephrotoxicity. Cisplatin can activate the pro-apoptotic B-cell lymphoma 2 (Bcl-2) family proteins, which induce defects on the mitochondria, causing the leakage of cytochrome c. This leakage forwards the multiprotein complex assembly that induces caspase-3 activation. Previous studies have shown that in addition to its anti-oxidant and radical-scavenging properties, melatonin exerts an anti-apoptotic effect on various types of cells (26). In this study, melatonin significantly inhibited caspase-3 activation and subsequent cleavage of PARP-1 in the cisplatin-treated mice. Apoptotic tubular cells were also reduced by melatonin. And melatonin significantly suppressed cisplatin-induced apoptosis in vitro, too. These results indicate that melatonin has an anti-apoptotic effect in cisplatin-induced tubular injury.
Although necrosis was originally thought to be a non-programmed and unregulated form of cell death, necroptosis, a newly identified type of cell death, has been considered a form of programmed necrosis (27).
It has been shown that a multiprotein complex composed of RIPK1 and RIPK3 plays a key regulatory role in initiating necroptosis. Caspase-8 mediates apoptotic cell death by stimulating downstream caspases like caspase-3. When caspase-8 is blocked, RIPK1 forwards necroptosis by interacting with RIPK3, which mediates the phosphorylation of mixed lineage kinase domain-like protein (MLKL). Phosphorylated MLKL forms oligomers and translocates to the plasma membrane, where it disrupts membrane integrity, resulting in cell lysis. Plasma membrane rupture in necroptosis leads to spilling of the intracellular contents, resulting in triggering of inflammation. Indeed, accumulating evidence offers that
inflammatory diseases (28,29). Recent studies demonstrated that necroptosis plays an important role in cisplatin-induced nephrotoxicity.
Expression of RIPK1 and RIPK3 were found to be increased in renal tubules after cisplatin treatment. In this study, the increased expression of RIPK1 and RIPK3 in the kidneys of cisplatin-treated mice was confirmed. This change was significantly attenuated by melatonin treatment. LDH release assay to evaluate the effect of melatonin on cisplatin-induced necrotic cell death in vitro was also performed. The result was that melatonin dose-dependently inhibited cisplatin-induced LDH release in cultured TCMK-1 cells. Cisplatin-induced upregulation of RIPK1 and RIPK3 was also significantly attenuated by melatonin.
Consistent with these findings, recent studies showed that melatonin ameliorated cardiac ischemia-reperfusion injury through suppression of RIPK3-dependent necroptosis. In addition, melatonin prevented carbon tetrachloride-induced liver fibrosis by inhibiting necroptosis-associated inflammatory signaling. In this study, melatonin also significantly attenuated NF-κB activation and expression of pro-inflammatory cytokines in cisplatin-treated mice. These anti-inflammatory effects of melatonin may be attributed, at least in part, to its ability to suppress necroptosis. These results suggest that melatonin inhibits cisplatin-induced necroptosis in renal tubular epithelial cells.
In conclusion, these data demonstrate that melatonin inhibits apoptosis and necroptosis which play critical roles in cisplatin-induced renal damage in cisplatin-treated mice and renal cells. These results indicate that melatonin may be a prospective therapeutic agent for the safeguarding of cisplatin-induced renal damage.
5. Summary
Melatonin is a hormone produced by the pineal gland in the brain and it has been reported that melatonin plays an important role in cisplatin-induced renal damage. In this study, the underlying mechanisms of melatonin were evaluated in the cisplatin-treated mice and cells. Melatonin significantly ameliorated renal dysfunction, increased expression of tubular injury markers, caspase-3 activation, apoptotic cell death, protein expression of key components of the molecular machinery for necroptosis, activation of NF-κB, and mRNA expression of pro-inflammatory cytokines in cisplatin-treated mice and cells. Taken together, these data demonstrated that the protective effects of melatonin against cisplatin-induced renal dysfunction and structural damages through dual suppression of apoptosis and necroptosis.
References
1. Pabla N, Dong Z: Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney International 2008; 73: 994-1007.
2. Sánchez-González PD, López-Hernández FJ, López-Novoa JM, Morales AI: An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Critical Reviews in Toxicology 2011; 41: 803-21.
3. Perše M, Večerić-Haler Ž: Cisplatin-induced rodent model of kidney injury: characteristics and challenges. BioMed Research International 2018; 2018: A1462802.
4. Tristão VR, Gonçalves PF, Dalboni MA, Batista MC, De Souza Durão Jr M, Monte JCM: Nec-1 protects against nonapoptotic cell death in cisplatin-induced kidney injury. Renal Failure 2012; 34:
373-7.
5. Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Zen FD, et al.: Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America 2013; 110: 12024-9.
6. Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, et al.: A role for tubular necroptosis in cisplatin-induced AKI. Journal of the American Society of Nephrology 2015; 26: 2647-58.
7. Wang S, Zhang C, Hu L, Yang C: Necroptosis in acute kidney injury: a shedding light. Cell Death and Disease 2016; 3: E2125.
8. Tristão VR, Pessoa EA, Nakamichi R, Reis LA, Batista MC, Monte JCM, et al.: Synergistic effect of apoptosis and necroptosis inhibitors in cisplatin-induced nephrotoxicity. Apoptosis 2016; 21:
51-9.
9. Hu W, Ma Z, Jiang S, Fan C, Deng C, Yan X, et al.: Melatonin:
the dawning of a treatment for fibrosis?. Journal of Pineal Research 2016; 60: 121-31.
10. Hara M, Yoshida M, Nishijima H, Yokosuka M, Ligo M, Ohtani-Kaneko R, et al.: Melatonin, a pineal secretory product with antioxidant properties, protects against cisplatin-induced nephrotoxicity in rats. Journal of Pineal Research 2001; 30: 129-38.
11. Parlakpinar H, Sahna E, Ozer MK, Ozugurlu F, Vardi N, Acet A:
Physiological and pharmacological concentrations of melatonin protect against cisplatin-induced acute renal injury. Journal of Pineal Research 2002; 33: 161-6.
12. Kilic U, Kilic E, Tuzcu Z, Tuzcu M, Ozercan IH, Yilmaz O, et al.:
Melatonin suppresses cisplatin-induced nephrotoxicity via activation of Nrf-2/HO-1 pathway. Nutrition and Metabolism 2013; 10: A7.
13. Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, et al.:
through SIRT3-dependent regulation of oxidative stress and apoptosis. Journal of Pineal Research 2017; 63: E12419.
14. Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, et al.: Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. Journal of Pineal Research 2018; 65: E12503.
15. Yang J, Yang Z, Li C, Wang Y, Yin Y, Liu M, et al.: Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis.
Journal of Molecular and Cellular Cardiology 2018; 125: 185-94.
16. Tahan V, Atug O, Akin H, Eren F, Tahan G, Tarcin O, et al.:
Melatonin ameliorates methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in rats. Journal of Pineal Research 2009; 46: 401-7.
17. Choi HS, Kang WJ, Lee SM: Melatonin attenuates carbon tetrachloride-induced liver fibrosis via inhibition of necroptosis.
Translational Research 2015; 166: 292-303.
18. Kim JY, Park JH, Kim K, Jo J, Leem J, Park KK: Pharmacological inhibition of caspase-1 ameliorates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation in Mice. Mediators of Inflammation 2018; 2018:
A6571676.
19. Deng B, Lin Y, Ma S, Zheng Y, Yang X, Li B, et al.: The leukotriene B4-leukotriene B4 receptor axis promotes cisplatin-induced acute kidney injury by modulating neutrophil recruitment. Kidney International 2017; 92: 89-100.
20. Barberino RS, Menezes VG, Ribeiro AEAS, Palheta RC, Jiang XJ, Smitz JEJ, et al.: Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biology of Reproduction 2017; 96: 1244-55.
21. Xu J, Sun S, Wei W, Fu J, Qi W, Manchester LC, et al.:
Melatonin reduces mortality and oxidatively mediated hepatic and renal damage due to diquat treatment. Journal of Pineal Research 2007; 42: 166-71.
22. Choi SH, Leem J, Lee IK: Protective effects of gemigliptin, a dipeptidyl peptidase-4 inhibitor, against cisplatin-induced nephrotoxicity in mice. Mediators of Inflammation 2017; 2017:
A4139439.
23. Newton K: RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends in Cell Biology 2015; 25: 347-53.
24. Meng HZ, Fu GH, Shen J, Shen KZ, Xu ZJ, Wang YM, et al.:
Ameliorative effect of daidzein on cisplatin-induced nephrotoxicity in mice via modulation of inflammation, oxidative stress, and cell death. Oxidative Medicine and Cellular Longevity 2017; 2017:
A3140680.
25. Salem N, Helmi N, Assaf N: Renoprotective effect of platelet-rich plasma on cisplatin-induced nephrotoxicity in rats. Oxidative Medicine and Cellular Longevity 2018; 2018: A9658230.
26. Park JH, Chung EJ, Kwon HJ, Im SS, Lim JG, Song DK:
Protective effect of melatonin on TNF-α-induced muscle atrophy in L6 myotubes. Journal of Pineal Research 2013; 54: 417-25.
27. Smith CC, Yellon DM: Necroptosis, necrostatins and tissue injury.
Journal of Cellular and Molecular Medicine 2011; 15: 1797-806.
28. Pasparakis M, Vandenabeele P: Necroptosis and its role in inflammation. Nature 2015; 517: 311-20.
29. Newton K, Manning G: Necroptosis and inflammation. Annual Review of Biochemistry 2016; 85: 743-63.
Melatonin Attenuates Cisplatin-Induced Nephrotoxicity through Dual Suppression of Apoptosis and Necroptosis
Kim, Jong Woo Department of Physiology
Graduate School Keimyung University
(Supervised by Professor Park, Jae Hyung)
Melatonin is a hormone produced by the pineal gland and is well
known to regulate the sleep-wake cycle. In addition, melatonin also has
beneficial effects such as anti-oxidant and anti-inflammatory properties
in various disease models. It has been reported that melatonin plays a
protective role against cisplatin-induced nephrotoxicity. However, the
mechanisms underlying the protective effect of melatonin on
cisplatin-induced kidney injury remains poorly understood. This study
showed that treatment of melatonin significantly ameliorated
cisplatin-induced kidney dysfunction and structural damages. Melatonin
inhibited cleaved caspase-3 and cleaved PARP-1 expressions in
cisplatin-treated mice. Necroptotic markers were markedly increased by
cisplatin treatment and these changes were also attenuated by melatonin.
In the cisplatin-treated mice, melatonin significantly inhibited expression
levels of phospho-NF-κB and pro-inflammatory cytokines. In renal
epithelial cell lines, melatonin also ameliorated cisplatin-induced cellular
apoptosis and necroptosis. These results suggest that melatonin protects
against cisplatin-induced renal dysfunction and structural damages
through dual suppression of apoptosis and necroptosis.
시스플라틴 유도 신독성에서 아포토시스와 네크롭토시스의 이중 억제에 의한 멜라토닌의 보호효과
김 종 우
계 명 대 학 교 대 학 원 의학과 생리학 전공 (지도교수 박 재 형)
멜라토닌은 뇌의 송과선에서 생산되는 호르몬으로 수면-각성 주기를 조
절하는 것으로 알려져 있다. 또한 멜라토닌은 다양한 질병 모델에서 항산화
및 항염증 작용과 같은 유익한 효과를 나타낸다. 멜라토닌은 시스플라틴에
의한 신독성으로부터 신장 조직을 보호하는 것으로 보고되었다. 하지만 그
기전은 아직까지 명확히 밝혀져 있지 않다. 본 연구에서는 시스플라틴을 투
여한 마우스 및 신장 세포주에서 멜라토닌의 효능을 확인하고 그 기전을
규명하였다. 동물실험에서 시스플라틴에 의한 혈액 요소질소 및 크레아티닌
의 혈청 수치 감소가 멜라토닌 처리에 의해 개선되었으며, 시스플라틴에 의
한 신장세포의 조직학적 변화도 멜라토닌 처리에 의해 개선되었다. 호중구
젤라티나제 결합 리포칼린과 신장 손상 분자-1과 같은 신장 관상세포의 손
상지표들의 발현이 시스플라틴에 의해 현저히 증가하였으며, 이는 멜라토닌
처리에 의해 억제되었다. 시스플라틴에 의해 신장 세포의 아포토시스와 네
크롭토시스 모두 현저히 증가하였으며, 아포토시스와 네크롭토시스 모두 멜
라토닌 처리에 의해 억제되었다. 시스플라틴에 의해 신장 조직의 염증성 사
이토카인들의 발현양도 현저히 증가하였으며, 멜라토닌 처리에 의해 유의하
게 감소되었다. 신장 세뇨관 상피 세포주를 이용한 실험에서도 시스플라틴
에 의한 아포토시스와 네크롭토시스 증가가 멜라토닌에 의해 모두 억제되
었다. 결론적으로 멜라토닌은 아포토시스와 네크롭토시스의 이중 억제를 통
하여 시스플라틴에 의한 신장의 기능이상과 구조적 손상을 예방하였다. 따
라서 멜라토닌은 시스플라틴에 의한 신독성을 예방할 수 있는 후보물질이
될 수 있다.