Maturity-onset diabetes of the young: update and perspectives on diagnosis and treatment
전체 글
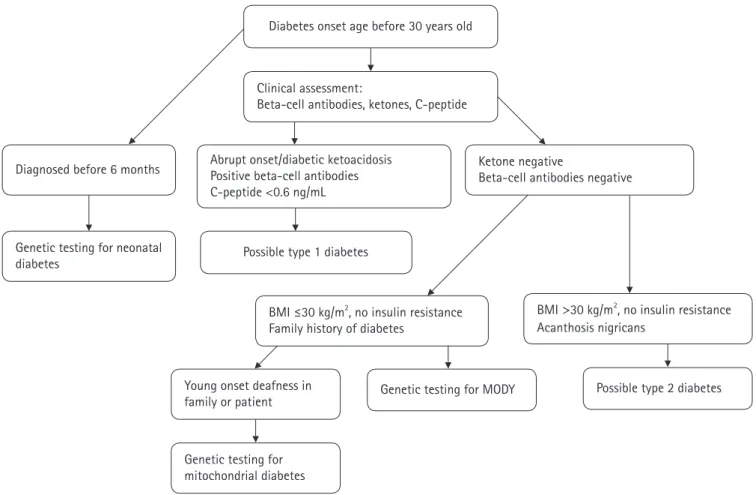
수치
관련 문서
웹 표준을 지원하는 플랫폼에서 큰 수정없이 실행 가능함 패키징을 통해 다양한 기기를 위한 앱을 작성할 수 있음 네이티브 앱과
_____ culture appears to be attractive (도시의) to the
Gasketed plate heat exchanger were introduced in the 1930s mainly for the food industries because of their ease of cleaning, and their design reached maturity in the 1960s
이하선의 실질 속에서 하악경의 후내측에서 나와 하악지의 내측면을 따라 앞으로 간다. (귀밑샘 부위에서 갈라져 나와
The index is calculated with the latest 5-year auction data of 400 selected Classic, Modern, and Contemporary Chinese painting artists from major auction houses..
2) Serum Cortisol, ACTH 3) Serum TSH and Free T4 4) Serum Prolactin and GH 5) Serum 25OHD and PTH.. What is the most common laboratory. abnormality expected in this
1 John Owen, Justification by Faith Alone, in The Works of John Owen, ed. John Bolt, trans. Scott Clark, "Do This and Live: Christ's Active Obedience as the
Students with family obligations and the revival of individual success is complicated on sail.. Second image of the sea is energy of young and