Effect of Botulinum Toxin A Injection into the Salivary Glands for Sialorrhea in Children with Neurologic Disorders
전체 글
수치
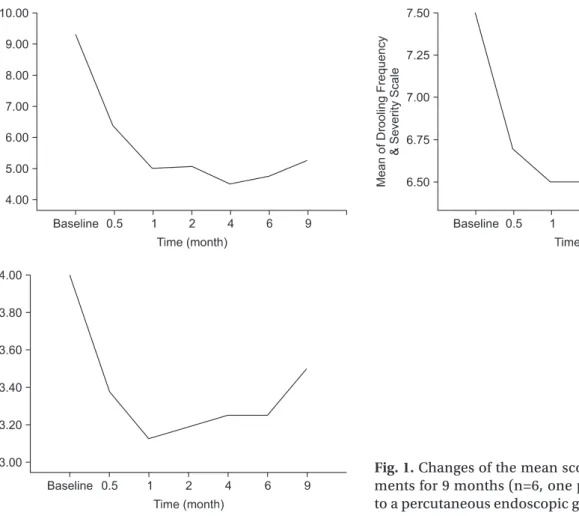
관련 문서
♦ A foreign country may subsidize the export of a good that the US also exports, which will reduce its price in world markets and decrease the terms of trade for the US.
with the experimental C versus t data. If the fit is unsatisfactory, another rate equation is guessed and tested. Integral method is especially useful for fitting simple
The index is calculated with the latest 5-year auction data of 400 selected Classic, Modern, and Contemporary Chinese painting artists from major auction houses..
1 John Owen, Justification by Faith Alone, in The Works of John Owen, ed. John Bolt, trans. Scott Clark, "Do This and Live: Christ's Active Obedience as the
As a results, the SDF showed the greatest remineralization effect among.. The fluoride varnish and tape showed similar remineralizing effect. Only in the SDF group, a difference
Methods: I conducted a retrospective analysis of clinical records of children aged between 4 and 19 years who were treated with flunarizine for headache at
A and E, In control group, a small amount of new bone was observed at the margin of bone defect (40×); B and F, In experimental group 1, a large amount of new bone was formed
3 Mean percentages of bone implant contact ratio in the control group and experimental groups at 6 and 12 weeks after placement of the