관련 문서
It also suggest that cell proliferation inhibits by a novel signal transduction for adenosine effect in human FaDu hypopharynx squamous cell carcinoma cells....
Frequency of conidial germination (A) and appressorium formation (B) of Colletotrichum orbiculare and callose formation of plant cells (C) at the
Effects of pulse frequency of low-level laser thrapy (LLLT)on bone nodule formation in rat calvarial cells.. Low-level laser therapy stimulats
Determining optimal surface roughness of TiO₂blasted titanium implant material for attachment, proliferation and differentiation of cells derived from
Histologic evaluation of early human bone response to different implant surfaces2. Histologic evaluation of human bone integration on machined and
Results: The human breast cancer cell subline MCF-7/MX5 cells selected in the presence of 5 µg/ml mitoxantrone (MX) were more resistant to MX (15.7... Western blot and
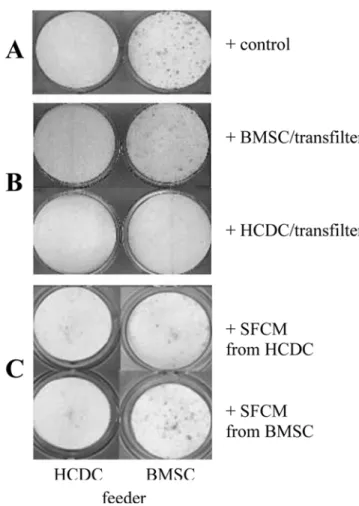
Approved clinical use of bone marrow stem cells for myocardial infarction treatment... Cardiac
4 > Effects of Taro on COX-2 expression and iNOS expression(hot water) in human thyroid cancer cells. The cells were pretreated for 48hours with either