Introduction
Patients with diabetes often suffer from diabetic polyneuropathy (DPN) and carpal tunnel syndrome (CTS). 1-3 DPN, which is defined as chronic, symmetric, length-dependent sensorimotor polyneuropathy, is known to frequently occur in patients with diabetes, thus
당뇨병환자에서 당뇨병성신경병증과 손목터널증후군의 임상적 차이
양서연, 김대열
울산대학교 의과대학 서울아산병원 재활의학과
Clinical Differences of Diabetic Polyneuropathy or Carpal Tunnel Syndrome in Patients with Diabetes
Seoyon Yang, Dae Yul Kim
Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
Received February 6, 2015
Revised (1st) April 14, 2015, (2nd) May 15, 2015, (3rd) May 19, 2015 Accepted May 19, 2015
Corresponding Author: Dae Yul Kim
Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Asanbyeongwon-gil 86, Songpa-gu, Seoul 138- 736, Korea.
Tel: 82-2-3010-3800, Fax: 82-2-3010-6964, E-mail: dykimsmart@gmail.com
Objective: To investigate whether there are clinical differences in patients with diabetic peripheral polyneuropathy (DPN)
or carpal tunnel syndrome (CTS).Method: 587 patients with diabetes mellitus who had an electrodiagnostic study to investigate whether they had DPN or
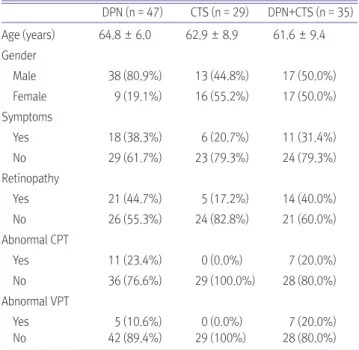
CTS were enrolled. The patients were divided into DPN, CTS, DPN+CTS groups. General characteristics, diabetes-related factors, and associated complications were compared between these two groups.Results: Of the 587 diabetic patients, 111 patients had DPN, CTS or both. Among 111 patients, 47 had DPN (42.3%), 29
had CTS (26.1%) and 35 had both (31.5%). The results showed that the duration of diabetes was associated with DPN.Patients who had both DPN and CTS had higher levels of Hb1Ac, PP2, albumin/creatinine ratio, and longer duration of diabetes than the patients who had only DPN or CTS.