INTRODUCTION
Cervical cancer is one of the most common female cancers worldwide and is also the most common cancer of female genital tract in Korea.1 Although there have been a decreased incidence and increased early detection of cervical cancer mainly due to the well-organized cytology- based screening programs especially in developed countries,2 locally advanced cervical cancer comprises a significant proportion of the total patients with cervical cancer, par- ticularly in developing countries.3 Radiation therapy has
been a main treatment modality for locally advanced cervical cancer.
Various factors have been introduced as prognostic factors for cervical cancer including clinical stage, nodal involvement, tumor size, depth of stromal invasion, adeno- carcinoma histology, microvessel density, tumor hypoxia, lymph-vascular space invasion, hemoglobin level, and interstitial tumor pressure.4-7 Recently, it has been reported that cyclooxygenase (COX)-2 expression in carcinoma of the cervix correlates with lymph node involvement in patients with stage IB disease treated with radical hysterectomy, and with diminished survival in patients treated with radiation therapy.8,9 Now, high expression of COX-2 in cervical cancer has been under massive investi- gation on a role as a prognostic indicator.
COX is a rate-limiting enzyme in producing eicosanoids and has two isoforms, COX-1 and COX-2. COX-1 is
Cyclooxygenase expressions and response to radiation therapy in uterine cervix cancer
Yong-Tark Jeon1, Sang-Soo Seo2, Jae Weon Kim1, Noh-Hyun Park1, Soon-Beom Kang1, Hyo-Pyo Lee1, Yong-Sang Song1
Department of Obstetrics and Gynecology1, Cancer Research Institute, College of Medicine, Seoul National University, Seoul, Center for Uterine Cancer2, National Cancer Center, Ilsan, Korea
Objective:The aim of this study is to clarify the relationship between COX expressions and radioresistance in cervical cancer.
Methods:Patients with cervical cancer treated by primary radiotherapy were selected from the tumor registry of our institution.
According to the response to radiotherapy during and after a month of radiation, poor responder and good responder was defined.
Immunohistochemical staining was performed by the ABC method using formalin-fixed, paraffin-embedded tissue sections and monoclonal anti-COX-1, 2 antibodies. Correlation of COX expression and response to radiation was analyzed. Cell lines derived from human cervical tumors were used: HeLa, HT3, and C33A. Using western blot, COX-1, 2 expressions were identified in each cell line. The sensitivity of the cervix cancer cells to radiation was measured using a clonogenic assay.
Results:COX-1 and COX-2 expressions were higher in poor responders than good responders. The difference of COX-1 expression between two groups had marginal statistical significance (p=0.099, Fisher’s exact test) and COX-2 expression was significantly higher in poor responders (p=0.034, Fisher’s exact test). In the clonogenic assay, survival fraction of HeLa and HT-3 cell lines, which have COX-1 and COX-2 activity, was significantly higher than C33A cell line which has no COX activity (p<0.001).
Conclusion:Our results suggest that the expression of COX in cervical cancer might be deeply associated with the effect of radiation therapy.
Key Words : Cervix cancer, Cyclooxygenase, Radiation
논문접수일:2006년 2월 17일 채택일:2006년 4월 6일 교신저자:송용상, 110-744 서울시 종로구 연건동 28
서울대학교 의과대학 산부인과학교실 전화:02-2072-2822․전송:02-762-3599 E-mail:yssong@snu.ac.kr
본연구는 서울대학교병원 일반연구비(과제번호: 04-2005-045-0) 지
원으로 수행되었음.
constitutively expressed in various normal cells and is involved in the maintenance of physiologic conditions. On the other hand, COX-2 is induced during inflammation and by various mitogens, such as, growth factors and cytokines.10 It has been proposed that COX-2 may regulate cell proliferation, mitosis, cell adhesion, apoptosis, immune surveillance and/or angiogenesis during carcinogenesis.11-16 Expression of COX-2 is associated with metastasis and poor prognosis in several malignancies.8,17-19 Furthermore several studies show that COX-2 expression may be associated with resistance to radiation and chemotherapeutic agents.9,20,21 In addition, recent report indicate that COX-1 up-regulation modulates the expression of factors that may act in an autocrine/paracrine manner to enhance and sustain tumori- genesis in neoplastic cervical epithelial cells.22
Although COX inhibitors are not used in cancer patients at present, it will be possible that non-steroidal anti- inflammatory drugs (NSAIDs) may be used routinely as a simple, cheap, and safe radiosensitizer, if the evidences for the relation of COX and radiation were sufficiently accu- mulated. Therefore the study to clarify the relationship of COX and radioresistance of cervix cancer is an interesting and valuable subject in the field of gynecologic oncology.
MATERIALS AND METHODS
1. Patients
Patients with cervical cancer treated by primary radiation therapy were selected from the tumor registry of Department of Therapeutic Radiology, Seoul National University Hospital from 1992 to 1997. Poor response to radiation was defined as follows; disease progression during radiation or no tumor regression after a month of radiation. Good response to radiation was that complete tumor regression during radiotherapy. For evaluation, weekly gynecologic examination was done during radiation therapy.
Among the stage IIA or higher staged uterine cervical cancer patients who had been treated with radical radiation therapy, 17 patients showed poor response to radiation. Of these patients, six patients, whose biopsy specimens were
not available, were excluded, and remaining 11 patients (poor responder) were enrolled in this study. All patients in this group had histology of squamous cell carcinoma. For comparison, good response group were selected. Eleven patients (good responder) similar to poor responder in terms of stage and histology were selected.
The patients in poor response group were treated as follows; seven patients were treated with radiation therapy alone, and four patients were previously treated with neoadjuvant chemotherapy but revealed progressive disease.
In all cases, 50.4 Gy was given to the whole pelvis, but seven patients could not receive brachytherapy. Poor geome- try for brachytherapy due to no tumor shrinkage was the reason. Two patients were boosted with external beam radiotherapy with cone-downed fields.
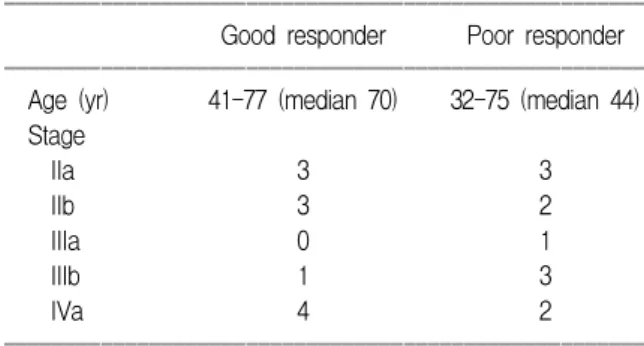
All patients in good response group were treated with radiation therapy alone. In all cases, 50.4 Gy was given to the whole pelvis, followed by one or two course of low-dose-rate brachytherapy with a total dose of 83-85 Gy to point A. If the patient had parametrial involvement, 6-10 Gy boost were given to the involved parametrium. Patient characteristics are summarized in Table 1.
2. Immunohistochemical staining of COX-1, 2
Immunohistochemical staining was performed by the ABC method using formalin-fixed, paraffin-embedded tissue sections as described previously.23 Cervix cancer tissues were reacted with anti-COX-2 primary antibody (Transduc- tion Lab., Lexington KY, USA) and with anti-COX-1 antibody (Santa Cruz Biotechnology, CA, USA) separately.
Table 1. Patient characteristics
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ Good responder Poor responder ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
Age (yr) 41-77 (median 70) 32-75 (median 44) Stage
IIa 3 3
IIb 3 2
IIIa 0 1
IIIb 1 3
IVa 4 2
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
All slides were incubated with biotinylated link antibody (DAKO A/S, Copenhagen, Denmark) and finally with avidin/biotinylated horseradish peroxidase solution. The samples were exposed to diaminobenzidine and counter- stained with Mayer’s hematoxylin, and mounted in Per- mount (Fisher Scientific, Fair Lawn, USA). The percentage of cells expressing COX-2 and COX-1 was estimated by dividing the number of positively stained cells by the total number of cells per high-power field. Tumor sections were classified as positive staining if percentage of immuno- stained tumor cells was higher than median value (30% in COX-1 and 10% in COX-2).
3. Western blot and clonogenic assay
Cell lines derived from human cervical tumors were used:
HeLa, HT3, and C33A. Cell lines were obtained from American Type Culture Collection (Manassas, VA, USA) and were maintained as exponentially growing monolayers in Dulbecco’s Modified Eagle Medium (DMEM) and RPMI 1640 (Sigma-Aldrich, St. Louis, MO, USA) containing 5%
fetal bovine serum.
To determine COX levels in cervical cancer cell lines, cells were trypsinized, washed with phosphate-buffered saline (PBS), and the cell pellet resuspended in 50μl lysate buffer [1×Protease Inhibitor Cocktail (Roche Molecular Biochemicals, Indianapolis, IN, USA) in RIPA buffer (150 mM NaCl, 0.1% SDS, 50 mM Tris, pH 8.0, 1% Triton X-100)]. The suspension was placed on ice for 20 min and then centrifuged at 4oC for 20 min at 13,000 rpm, after which the supernatant was recovered. Protein concentrations were determined using the protein assay kit bicinchoninic acid (Pierce Biotechnology Inc., Rockford, IL, USA). Twenty μg of cell lysate protein were separated on 12% SDS- PAGE polyacrylamide gel with Laemmli buffer and the proteins then transferred to an Immobilon-P membrane (Millipore Corp., Bedford, MA, USA). After the transfer, the membrane was blocked with 5% nonfat milk in TBS-T (0.05% Tween 20, 10 mM Tris-HCl pH 8.0, 150 mM NaCl) for 1 hr and then washed twice with TBS-T for 10 min each time. PGHS-1 and 2, IgG fraction of a mouse anti-COX-1
and -2 antibody (Oxford Biomedical Research Inc., Oxford, MI, USA), were then added at a 1:500 dilution for 2 hrs.
The membrane was washed 3 times with TBS-T for 10 min each. The secondary goat anti-mouse IgG alkaline phosphatase conjugate (Bio-Rad Lab., Hercules, CA, USA) was added at a 1:1,000 dilution for 2 hrs. The membrane was washed 3 times in TBS-T and detection was performed using an enhanced chemiluminescence (ECL) system (Amersham Bio- science, Arlington Heights, IL, USA).
The sensitivity of the cervix cancer cells to radiation was measured using a clonogenic assay. Cultured cells were exposed to SC-236 (10μM or 50μM) for 3 days. Then the cells were irradiated with graded doses (0, 2, 4, or 8 Gy) of γ-rays using a 137Cs source (3.7 Gy/min). Colony- forming ability of cells was assayed by re-plating them in specified numbers into 60 mm dishes in drug-free medium.
After 14 days of incubation, the cells were stained with 0.5% crystal violet in absolute ethanol, and colonies with more than 50 cells were counted. Radiation survival curves were plotted after normalizing for the cytotoxicity induced by SC-236 alone. Clonogenic survival curves were con- structed from three independent experiments by fitting the average survival levels using least-squares regression by the linear-quadratic model.
RESULTS
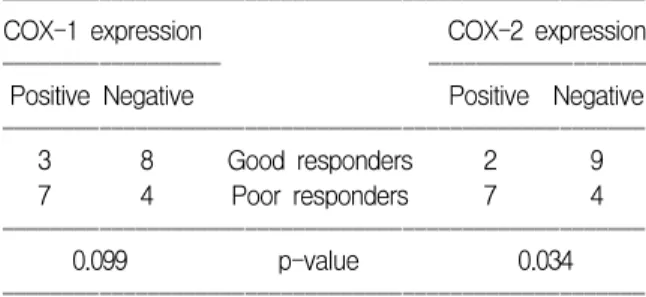
COX-1 and COX-2 expressions were higher in poor responders than good responders as shown in Table 2. The difference of COX-1 expression between two groups had marginal statistical significance (p=0.099, Fisher’s exact
Table 2. Results of the immunohistochemical staining ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
COX-1 expression COX-2 expression
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
Positive Negative Positive Negative
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
3 8 Good responders 2 9
7 4 Poor responders 7 4
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
0.099 p-value 0.034
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ
test). However, COX-2 expression was significantly higher in poor responders (p=0.034, Fisher’s exact test).
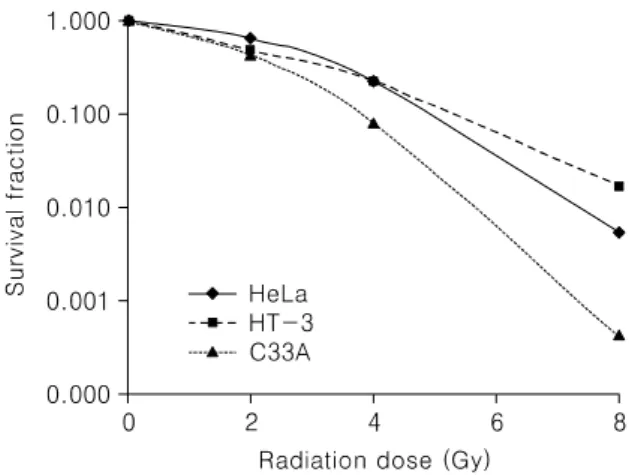
As shown in Fig. 1, C33A cell line did not express COX-1 and COX-2. On the contrary, HeLa and HT-3 cell lines had both COX-1 and COX-2 expression. In the clonogenic assay, as shown in Fig. 2, survival fraction of HeLa and HT-3 cell lines, which have COX-1 and COX-2 activity, was significantly higher than C33A cell line (p<
0.001).
DISCUSSION
Our results suggest that the expression of COX in cervical cancer might be deeply associated with the effect of radiation therapy. In the immunohistochemical staining study, patients who were resistant to radiation therapy had high COX expression. In additional experiment using clonogenic assay, COX expressing cell lines, HeLa and HT-3, were more resistant to ionizing radiation than C33A which has no COX activity. These results support the association of COX expression with radioresistance in cervix cancer.
As described in Introduction section, it has been proposed that COX-2 may play an important role in carcinogenesis, metastasis, poor prognosis, and resistance to radiation.
Recent clinical study on patients with cervical cancer who underwent radiation therapy also revealed the relationship of COX-2 expression with poor prognosis.9,24 Recently, Sales et al. showed that overexpression of COX-1 in HeLa cells up-regulates expression of COX-2 and prostaglandin
E synthase concomitant with increased prostaglandin E2 production.22 In addition, Narko et al. showed that COX-1 overexpression in endothelial cells implanted in mice was associated with enhanced tumorigenecity.25 Taken together, COX-1 might be important in cell survival and/or pro- liferation as COX-2 did.
Numbers of studies showed that COX-2 is increased in premalignant and malignant tissues of human. These studies cover gastrointestinal tract, liver, pancreas, head and neck, lung, breast, urinary bladder, uterine cervix, endometrium, and skin.15,26-35 Although the exact functional role(s) of increased COX-2 in tumor tissues have not been fully elucidated yet, there have been several proposed mecha- nisms on the role of tumor-derived prostanoids; angio- genesis,36 cell proliferation,37 resistance to apoptosis,38 and metastatic potential and/or invasiveness of a tumor.39 On the basis of these works, lots of studies were initiated to prevent cancer and to increase an efficacy of conventional cancer therapy with the use of COX inhibitor in general population and cancer patients. Regarding radiotherapy with COX inhibitor, Liao et al. reported encouraging results in non-small cell lung cancer patients using celecoxib.40 Cu- rrently, the Radiation Therapy Oncology Group (www.rtog.
org) is conducting clinical trials in cervix cancer, lung cancer and brain tumors, using inhibitors of COX-2 in combination with chemotherapy and radiation therapy.
Fig. 1. Western blotting of COX-1 and COX-2 in three cell lines. HeLa and HT-3 cell lines had both COX-1 and COX-2 expression but C33A cell line had neither.
Fig. 2. Clonogenic survival curve of three cell lines.
Survival fraction of HeLa and HT-3 cell lines, which have COX-1 and COX-2 activity, was significantly higher than C33A cell line (p<0.001).
Although cervix cancer is a radiocurable disease, there have been substantial concerns over frequent treatment failure of radiotherapy in locally advanced disease. Recent large randomized clinical trials have shown survival benefit of the concurrent use of cisplatin-based chemotherapy with radiation in patients with locally advanced disease or high-risk settings. In terms of additional chemotherapeutic agent during radiotherapy can cause more serious morbidity and increased cost, there has been incessant need for cheap and safe radiosensitizer. COX inhibitors are promising candidates for these purposes. However exact action mecha- nism(s) of these drugs to cancer cells is largely unknown at present and precise role(s) of COX, moreover, is only partially understood. We think that this study, although need further investigation, suggested small clue for verifying the enzymes’ role in radiosensitivity modulation and for devel- oping appropriate radiosensitizer of cervix cancer.
REFERENCES
1. Department of Gynecologic Tumor, Korean Society of Obstetrics and Gynecology. Annual report of Gynecologic Cancer Reg- istry Program in Korea for 2002. Korean J Obstet Gynecol 2004; 47: 1029-70.
2. Screening for squamous cervical cancer: Duration of low risk after negative results of cervical cytology and its implication for screening policies. IARC Working Group on evaluation of cervical cancer screening programmes. Br Med J (Clin Res Ed) 1986; 293: 659-64.
3. Kim RY, Alvarez RD. Recent developments in chemoradio- therapy for locally advanced cancer of the cervix. Oncology (Huntingt) 2000; 14: 1327-31.
4. DeVita Jr VT, Hellman S, Rosenberg SA. Cancer: Principles and practices of oncology. 7th ed. Philadelphia: Lippincott Williams & Wilkins;2005.
5. Oka K, Suzuki Y, Nakano T. Expression of p27 and p53 in cervical squamous cell carcinoma patients treated with radio- therapy alone: Radiotherapeutic effect and prognosis. Cancer 2000; 88: 2766-73.
6. Lyng H, Sundfor K, Trope C, Rofstad EK. Disease control of uterine cervical cancer: Relationships to tumor oxygen tension, vascular density, cell density, and frequency of mitosis and apoptosis measured before treatment and during radiotherapy. Clin Cancer Res 2000; 6: 1104-12.
7. Milosevic MF, Fyles AW, Wong R, Pintilie M, Kavanagh MC, Levin W, et al. Interstitial fluid pressure in cervical carcinoma:
Within tumor heterogeneity, and relation to oxygen tension.
Cancer 1998; 82: 2418-26.
8. Ryu HS, Chang KH, Yang HW, Kim MS, Kwon HC, Oh KS.
High cyclooxygenase-2 expression in stage IB cervical cancer with lymph node metastasis or parametrial invasion. Gynecol Oncol 2000; 76: 320-5.
9. Gaffney DK, Holden J, Davis M, Zempolich K, Murphy KJ, Dodson M. Elevated cyclooxygenase-2 expression correlates with diminished survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys 2001; 49:
1213-7.
10. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 1998; 38: 97-120.
11. Watkins DN, Lenzo JC, Segal A, Garlepp MJ, Thompson PJ.
Expression and localization of cyclo-oxygenase isoforms in non-small cell lung cancer. Eur Respir J 1999; 14: 412-8.
12. Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia 2001; 3: 53-61.
13. Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res 2001; 7: 861-7.
14. Sakuma K, Fujimori T, Hirabayashi K, Terano A. Cyclo- oxygenase (COX)-2 immunoreactivity and relationship to p53 and Ki-67 expression in colorectal cancer. J Gastroenterol 1999; 34: 189-94.
15. Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 1998; 58: 4997-5001.
16. Williams CS, Mann M, DuBois RN. The role of cyclooxy- genases in inflammation, cancer, and development. Oncogene 1999; 18: 7908-16.
17. Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK.
Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer 2001; 91: 894-9.
18. Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, et al. Depth of invasion parallels increased cyclooxygenase- 2 levels in patients with gastric carcinoma. Cancer 2001; 91:
1876-81.
19. Shirahama T, Arima J, Akiba S, Sakakura C. Relation between cyclooxygenase-2 expression and tumor invasiveness and patient survival in transitional cell carcinoma of the urinary bladder. Cancer 2001; 92: 188-93.
20. Ferrandina G, Ranelletti FO, Lauriola L, Fanfani F, Legge F, Mottolese M, et al. Cyclooxygenase-2 (COX-2), epidermal growth factor receptor (EGFR), and Her-2/neu expression in ovarian cancer. Gynecol Oncol 2002; 85: 305-10.
21. Ratnasinghe D, Daschner PJ, Anver MR, Kasprzak BH, Taylor PR, Yeh GC, et al. Cyclooxygenase-2, P-glycoprotein-170 and drug resistance; Is chemoprevention against multidrug resis- tance possible? Anticancer Res 2001; 21: 2141-7.
22. Sales KJ, Katz AA, Howard B, Soeters RP, Millar RP, Jabbour HN. Cyclooxygenase-1 is up-regulated in cervical carcinomas:
Autocrine/paracrine regulation of cyclooxygenase-2, prosta- glandine receptors, and angiogenic factors by cyclooxy- genase-1. Cancer Res 2002; 62: 424-32.
23. Kim MH, Seo SS, Song YS, Kang DH, Park IA, Kang SB, et al. Expression of cyclooxygenase-1 and -2 associated with expression of VEGF in primary cervical cancer and at metastatic lymph nodes. Gynecol Oncol 2003; 90: 83-90.
24. Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ, Chang SK, et al. Overexpression of cyclooxygenase-2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer 2002; 95: 531-9.
25. Narko K, Ristimaki A, MacPhee M, Smith E, Haudenschild CC, Hla T. Tumorigenic transformation of immortalized ECV endothelial cells by cyclooxygenase-1 overexpression. J Biol Chem 1997; 272: 21455-60.
26. Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 1995; 55: 3785-9.
27. Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma.
Cancer Res 1997; 57: 1276-80.
28. Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, et al. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer.
Carcinogenesis 1998; 19: 723-9.
29. Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclo- oxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst 1998; 90: 455-60.
30. Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res 1999; 59:
991-4.
31. Kondo M, Yamamoto H, Nagano H, Okami J, Ito Y, Shimizu
J, et al. Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin Cancer Res 1999;
5: 4005-12.
32. Mohammed SI, Knapp DW, Bostwick DG, Foster RS, Khan KN, Masferrer JL, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res 1999; 59: 5647-50.
33. Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res 1999; 59: 987-90.
34. Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, et al. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res 2001; 7: 429-34.
35. Jeon YT, Kang S, Kang DH, Yoo KY, Park IA, Bang YJ, et al. Cyclooxygenase-2 and p53 expressions in endometrial cancer. Cancer Epidemiol Biomarkers Prev 2004; 13: 1538-42.
36. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998; 93: 705-16.
37. Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: A novel mechanism for promoting colon cancer growth and gastro- intestinal hypertrophy. Nat Med 2002; 8: 289-93.
38. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995; 83: 493-501.
39. Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expres- sion in human colon cancer cells increases metastatic potential.
Proc Natl Acad Sci USA 1997; 94: 3336-40.
40. Liao Z, Komaki R, Milas L, Yuan C, Kies M, Chang JY, et al. A phase I clinical trial of thoracic radiotherapy and concurrent celecoxib for patients with unfavorable perfor- mance status inoperable/unresectable non-small cell lung cancer. Clin Cancer Res 2005; 11: 3342-8.
자궁경부암에서 싸이클로옥시게나제의 발현과 방사선치료 효과와의 관계
서울대학교 의과대학 산부인과학교실1, 국립암센터2
전용탁1․서상수2․김재원1․박노현1․강순범1․이효표1․송용상1
목적:자궁경부암에서 싸이클로옥시게나제의 발현과 방사선치료에 대한 반응성 사이의 관련을 알아보고자 하였다.
연구방법:자궁경부암으로 방사선치료를 받은 환자들 중 방사선치료에 대한 반응이 좋지 않았던 11명의 환자와 방 사선치료에 대한 반응이 좋았던 11명의 환자를 선택하였고, 보관된 암 조직에 대하여 싸이클로옥시게나제-1,2의 발현 을 면역조직화학염색을 통하여 알아보았다. 또한, 세 가지 자궁경부암 세포주(HeLa, HT-3, C33A)에 대하여 Western blot을 통해 싸이클로옥시게나제-1,2의 발현을 확인하고 싸이클로옥시게나제의 발현 차이에 따라 방사선 감수성의 변 화가 있는지 clonogenic assay를 통하여 알아보았다.
결과:면역조직화학염색 결과 싸이클로옥시게나제-1의 발현은 방사선치료에 대한 반응이 좋지 않았던 군에서 많이 발현되었으나 통계적 유의성은 높지 않았다(p=0.099, Fisher’s exact test). 싸이클로옥시게나제-2의 발현은 방사선치료에 대한 반응이 좋지 않았던 군에서 유의하게 많이 발현되었다(p=0.034, Fisher’s exact test). Western blot에서 HeLa와 HT-3 세포주는 싸이클로옥시게나제-1,2가 모두 발현되는 것을 알 수 있었고, C33A 세포주는 싸이클로옥시게나제의-1,2의 발 현이 없었다. Clonogenic assay에서는 HeLa와 HT-3 세포주의 생존 분율이 C33A 세포주보다 유의하게 높았다(p<0.001).
결론:본 연구를 통하여 자궁경부암에서 싸이클로옥시게나제의 발현은 방사선치료 자체에 대한 저항성과 깊은 관 련이 있을 가능성을 확인하였다.
중심단어:자궁경부암, 싸이클로옥시게나제, 방사선치료