Simvastatin as a Modulator of Tissue Remodeling through Inhibition of Matrix Metalloproteinase (MMP) Release from Human Lung Fibroblasts
전체 글
수치
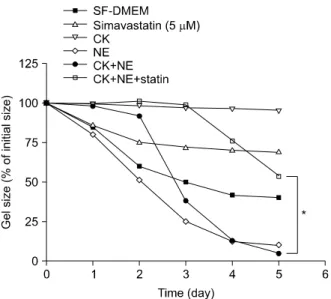
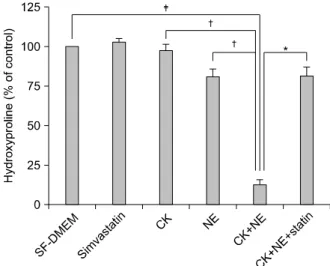
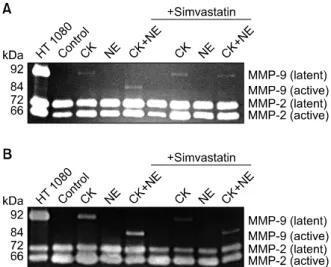
관련 문서
Concentration-Dependent inhibition of cell viability by adenosine in human oral fibroblast and FaDu human head and neck squamous cell carcinoma · · ·
As a result of the study, it was confirmed that only human resource competency had a positive(+) effect on the intercompany cooperation method of IT SMEs,
In this study, we investigated the effects of low-power CO 2 laser on proliferation on human gingival fibroblast cells so that determine laser
Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1.
The differences found in these concepts of human resources and HRD originate from their diverse subjects and objectives. In the narrow definition of the concept, the subjects of
As the importance of education through play in modern children's education is emphasized, this study aims to propose an art education program through play
In this study, we found that Pin1 plays a pivotal role in insulin- induced AP-1 activation through its interaction with p70S6K and prolongs activity of
Second, it was found that human rights education plays a controlling role in human rights sensitivity and human rights awareness (equal rights, right to