Reduced risk for chronic kidney disease after recovery from metabolic syndrome: A nationwide population- based study
Sehoon Park 1,2 , Soojin Lee 3,4 , Yaerim Kim 5 , Yeonhee Lee 3,4 , Min Woo Kang 3,4 , Kyungdo Han 6 , Hajeong Lee 3 , Jung Pyo Lee 4,7,8 , Kwon Wook Joo 3,4,7 , Chun Soo Lim 4,7,8 , Yon Su Kim 1,3,4,7 , Dong Ki Kim 3,4,7
1 Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Republic of Korea
2 Department of Internal Medicine, Armed Forces Capital Hospital, Seongnam, Republic of Korea
3 Department of Internal Medicine, Seoul National University Hospital, Seoul, Republic of Korea
4 Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea
5 Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Republic of Korea
6 Department of Medical Statistics, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
7 Kidney Research Institute, Seoul National University College of Medicine, Seoul, Republic of Korea
8 Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Republic of Korea
Background: Metabolic syndrome (MetS) is linked to various chronic comorbidities, including chronic kidney disease (CKD).
However, few large studies have addressed whether recovery from MetS is associated with reduction in the risks of such comorbidities.
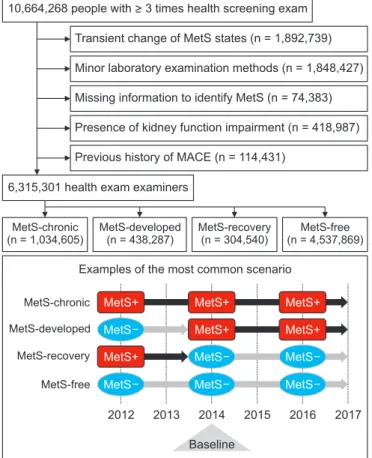
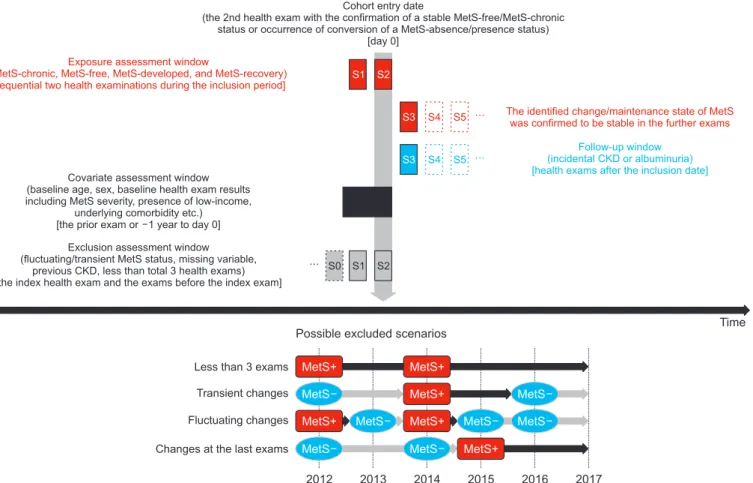
Methods: This nationwide population-based study in Korea screened 10,664,268 people who received national health screening ≥ 3 times between 2012 and 2016. Those with a history of major cardiovascular events or preexisting CKD were excluded. We classified study groups into four, according to the course of MetS state, as defined by the harmonizing criteria. The main study outcome was incidental CKD (estimated glomerular filtration rate < 60 mL/min/1.73 m 2 which was persistent until the last health exams). The study outcomes were investigated using multivariable logistic regression analysis, which was adjusted for clinical variables and the previous severity of MetS.
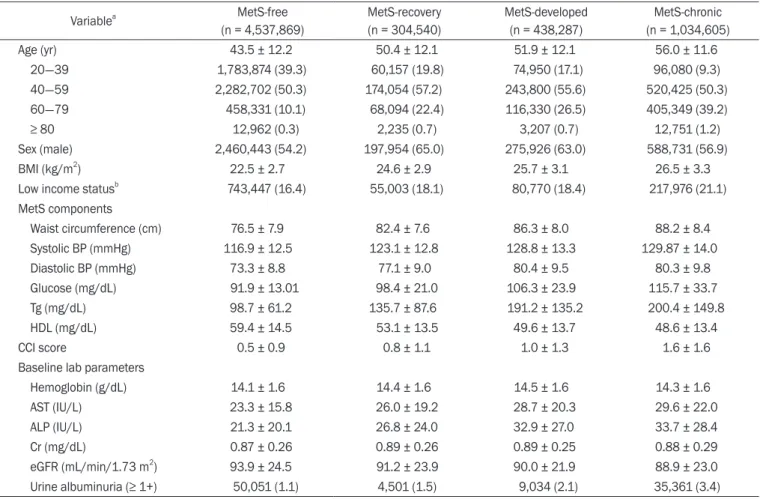
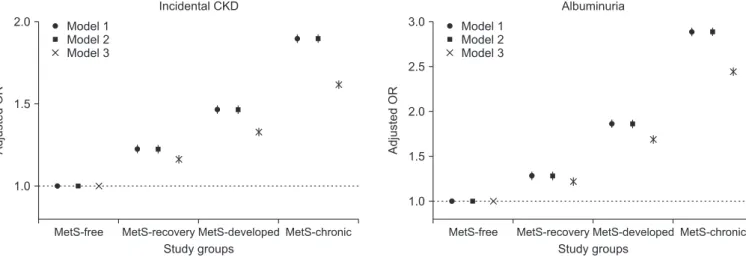
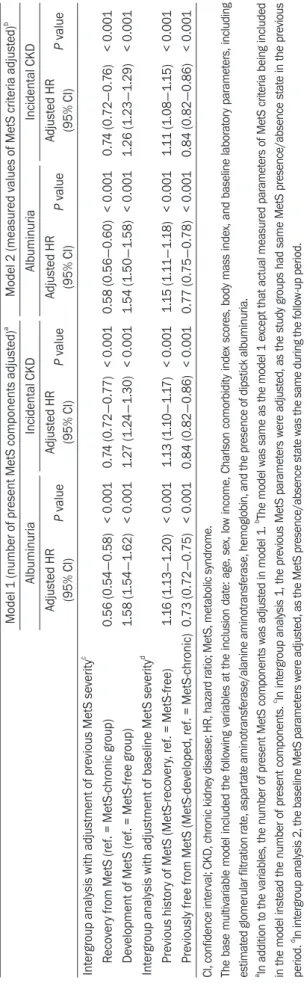
Results: Four study groups included 6,315,301 subjects: 4,537,869 people without MetS, 1,034,605 with chronic MetS, 438,287 who developed MetS, and 304,540 who recovered from preexisting MetS. Those who developed MetS demonstrated higher risk of CKD (adjusted odds ratio [OR], 1.26 [1.23-1.29]) than did those who did not develop MetS. In contrast, MetS- recovery was associated with decreased risk of CKD (adjusted OR, 0.84 [0.82-0.86]) than that in people with chronic MetS.
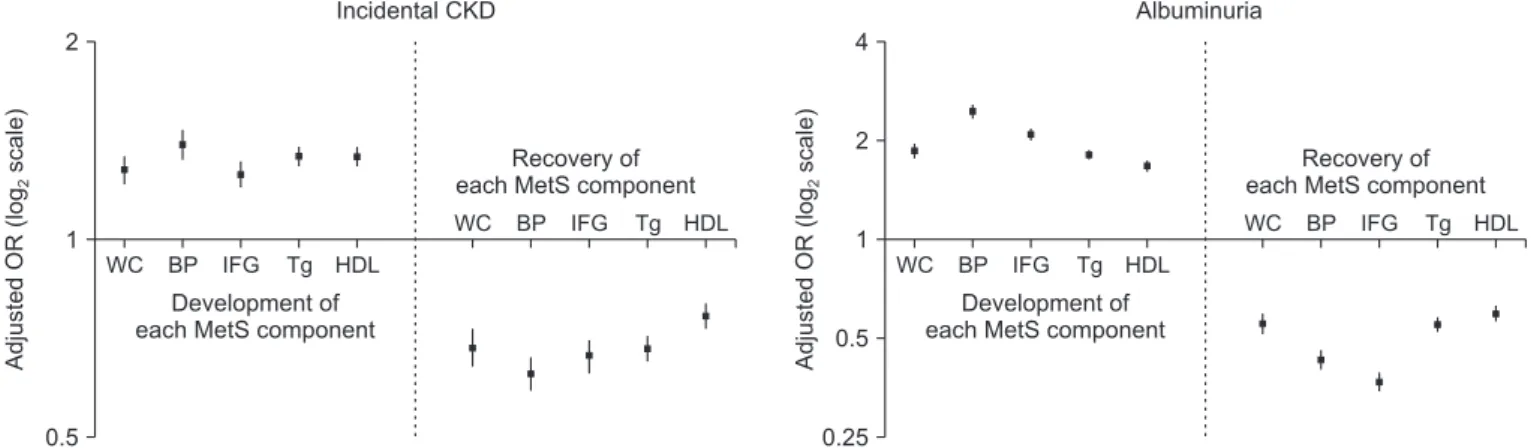
Among the MetS components, change in hypertension was associated with the largest difference in CKD risk.
Conclusion: Reducing or preventing MetS may reduce the burden of CKD on a population-scale. Clinicians should consider the clinical importance of altering MetS status for risk of CKD.
Keywords: Chronic kidney diseases, Diabetes mellitus, Dyslipidemia, Hypertension, Metabolic syndrome, Obesity Kidney Res Clin Pract 2020;39(2):180-191
pISSN: 2211-9132 • eISSN: 2211-9140 https://doi.org/10.23876/j.krcp.20.016
KIDNEY RESEARCH
AND CLINICAL PRACTICE
Received January 27, 2020; Revised March 13, 2020; Accepted March 15, 2020 Edited by Dong-Ryeol Ryu, Ewha Womans University, Seoul, Republic of Korea Correspondence: Dong Ki Kim
Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Republic of Korea.
E-mail: dkkim73@gmail.com.
Copyright © 2020 by The Korean Society of Nephrology
CC