Vol. 26, No. 8 (2016)
449
Microstructure and Biocompatibility of Porous BCP(HA/ β-TCP) Biomaterials Consolidated by SPS Using Space Holder
Kee-Do Woo 1† , Seung-Mi Kwak 1 , Tack Lee 1 , Seong-Tak Oh 1 and Jeong-Nam Woo 2
1Division of Advanced Material Engineering & RCAMD, Chonbuk National University, Jeonju 54896, Republic of Korea
2College of Veterinary Medicine, Chonbuk National University, Jeonju 54896 & National Institute of Food and Drug Safety Evaluation, Cheongju 28159, Republic of Korea
(Received May 12, 2016 : Revised July 12, 2016 : Accepted July 25, 2016)
Abstract
HA (hydroxyapatite)/β-TCP (tricalcium phosphate) biomaterial (BCP; biphasic calcium phosphate) is widely used as bone cement or scaffolds material due to its superior biocompatibility. Furthermore, NH4HCO3 as a space holder (SH) has been used to evaluate feasibility assessment of porous structured BCP as bone scaffolds. In this study, using a spark plasma sintering (SPS) process at 393K and 1373K under 20MPa load, porous HA/β-TCP biomaterials were successfully fabricated using HA/β-TCP powders with 10~30 wt% SH, TiH2 as a foaming agent, and MgO powder as a binder. The effect of SH content on the pore size and distribution of the BCP biomaterial was observed by scanning electron microscopy (SEM) and a microfocus X-ray computer tomography system (SMX-225CT). The microstructure observations revealed that the volume fraction of the pores increased with increasing SH content and that rough pores were successfully fabricated by adding SH.Accordingly, the cell viabilities of BCP biomaterials were improved with increasing SH content. And, good biological properties were shown after assessment using Hanks balanced salt solution (HBSS).
Key words
porous bioceramics, hydroxyapatite, tricalcium phosphate, biphasic calcium phosphate, space holder.1. Introduction
The rapid growth of the global population is leading to an increased demand for implants as biomaterials for the repair, reconstruction and replacements of damaged bone caused by diseases and accidents.
1)Ceramics used for implants as a biomaterial are termed ‘‘bio-ceramics’’.
2)Among bio-ceramics, hydroxyapatite(HA) has been widely used as bone substitutes due to superior biocompatibility with the bone. However, when HA is used as bone substitutes solely, HA restrains the recovery of defected bone because it remains in the body during recovery of the bone due to low bio-degradable rate. But β-TCP is a one of calcium phosphates and known as high bio- degradable materials in the body like magnesium. There- fore, a lot of researchers have been tried to fabricate BCP(HA + β-TCP; tri calcium phosphate) instead of HA or β-TCP cement.
3-4)On the other hand, porous-structure of biomaterials is also an important factor. Because, cir-
culation of body fluids into porous-structure is very important factor to restore the bone.
5)There are a lot of methods to fabricate the porous structure.
6-10)Among them, NH
4HCO
3as space holder(SH) was used in this study as a volatilization method. Volatilization of SH is a simple to make porous structures by sintering.
Thus, porous structured BCP biomaterials were fabri- cated using SH (space holder; NH
4HCO
3, TiH
2) by SPS (spark plasma sintering) to evaluate feasibility assessment as bone scaffolds.
2. Experimental Procedure
The HA and β-TCP powders were mixed 80 % to 20 % ratio (wt%) and the NH
4HCO
3(10, 20, 30 wt%) as space holder(SH) mixed with HA/ β-TCP(BCP) powders.
And TiH
2as a foaming agent(FA) and MgO as a binder were added in the mixed powders at 2 and 1 wt%, re- spectively as listed in Table 1. And prepared powders
†
Corresponding author
E-Mail : kdwoo@jbnu.ac.kr (K. D. Woo, Chonbuk Nat'l Univ.)
© Materials Research Society of Korea, All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creative-
commons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the
original work is properly cited.
were mixed for 24h by mechanical mixer(ABB ACS100).
The mixed powders were placed in a cylindrical graphite die (outside diameter: 35 mm, inside diameter: 10.5 mm, height: 40 mm) of the SPS equipment(Spark plasma sin- tering, Sumitomo). The chamber was evacuated 10
−2torr and a uniaxial pressure of 20MPa was applied. The powders were sintered by two steps at 393K for 5 minutes and heated up to 1373K for 5 minutes and instantly cooled in chamber. The pores distribution and morphology of samples were observed by field emission scanning electron microscope(FE-SEM: JMS-6400, Carl Zeiss), micro-focus X-ray device(SMX-225 CT, SHIMADZU).
The phase analysis was conducted by X-ray diffraction (XRD, Rigaku).
Hank’s balanced salt solution(HBSS) was prepared for immersion test. The sintered samples were soaked in HBSS at 37
oC for 1, 7, 15 and 30 days and the solutions were refreshed daily. A SEM equipped with an energy- dispersive X-ray spectrometer(EDS) was used to evaluate not only the surface morphologies of the samples after immersion in HBSS but also formation of apatite.
MTT assay was conducted using 5 × 10
4HOB(Human osteoblast) cells. After a period of 72h in incubator under 5%CO2 atmosphere, specimens were immersed into tetrazolium-based colorimetric(MTT) solution for 4h and then the specimens were read by ELISA(Enzume-Linked Immuno Sorbent Assay) leader. Cell attachments test was conducted using human osteoblast(hFOB) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) enriched with 10 % FBS (Gibco-Invitrogen, Carlsbad, CA. USA). 1 % penicillin and streptomycin (Sigma-Aldrich, St. Louis, MO, USA) in a 5 % CO2 humidified incubator at 34
oC Cells were maintained as a monolayer (80-90 % confluency) and used from sixth to ninth passage. The cells were seeded in 24 well plate at a density of 5 × 10
3cells/well and then incubated for 24h.
After incubation, cells were fixed with cold acetone for 2min and stained by DAPI(diamidino-2-phenylindole) staining method; which is one of fluorescent staining methods.
3. Results and Discussion
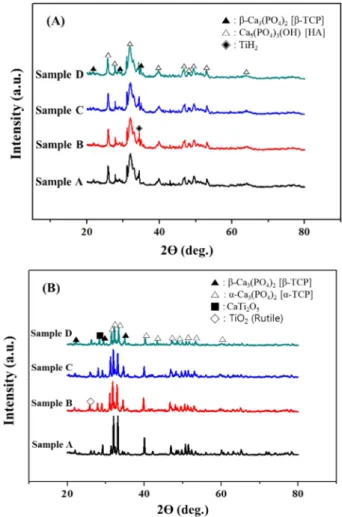
As a result of phase analysis(Fig. 1), a crystalline sintered BCP samples can be obtained through sintering and there
are new phases formed by sintering such as α-Ca
3(PO
4)
2[TCP], CaTi
2O
5, TiO
2due to interaction between HA and Table 1. The designed composition of samples(wt%).
Sample HA β-TCP NH
4HCO
3TiH
2MgO
A 79.2 19.8 X X 1
B 69.6 17.4 10 2 1
C 61.6 15.4 20 2 1
D 53.6 13.4 30 2 1
Fig. 1. The XRD results of HA/β-TCP biomaterials: (A) before sintering; (B) after sintering.
Fig. 2. 3D-CT images of HA/β-TCP biomaterials: (a) Sample A;
(b) Sample B; (c) Sample C; (d) Sample D.
TiH
2during sintering.
11-12)As shown in the CT images(Fig. 2), pore distribution and mean elongated pore size increased(466-525 μm;
Table 2) with increasing SH contents and elongated pores was observed. Elongated pores were caused by uniaxial pressure in a SPS furnace during sintering. By uniaxial pressure, pores were easily connected with near pores.
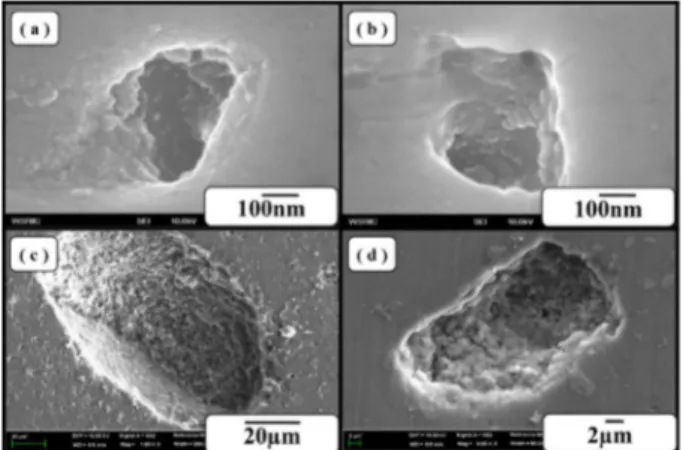
The pores on surface of the sintered BCP samples increased with increasing SH contents and large pores were successfully fabricated(Figs. 3, 4) by floating H
2gas on the surface from TiH
2.
12)Rough surfaces promote greater amounts of protein adsorption and efficiently stimulate more new bone formation than conventional
surface due to their high specific surface area.
13)After immersion test in HBSS, the new particles were formed after 7 days and covered on the surface as a layer after 30 days(Fig. 5). By EDS analysis of this layer(Table 3), oxygen, phosphorous and calcium are detected as main elements of layer. Those elements are main elements of apatite layer [Ca
5(PO
4)
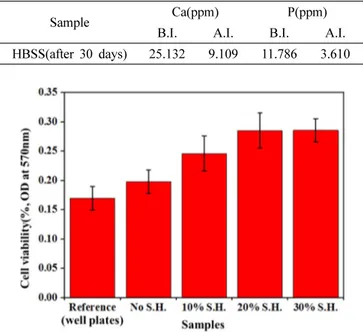
3(OH, F, Cl)]. In immersion test of sample D(SH added samples), apatite layer was also formed(Fig. 6). As a result of ICP analysis(Table 4), calcium and phosphorous ions decreased after immersion Table 2. The mean elongated pore size of HA/β-TCP biomaterials.
Sample Mean Pore Size( μm)
A 2
B 466
C 492
D 525
Fig. 3. The surface images of HA/β-TCP biomaterials: (a) Sample A; (b) Sample B; (c) Sample C; (d) Sample D.
Fig. 4. The higher magnification images of pores: (a), (b) Sample A; (c), (d) Sample D.
Fig. 5. The SEM results of Sample A through immersion in HBSS.
Table 3. The EDS result of sample A after immersion test for 30 days.
Element Wt% At%
O 68.95 82.53
Na 0.18 0.15
Mg 2.19 1.72
P 13.48 8.34
K 0.25 0.12
Ca 14.95 7.14
Tatal 100 100
Fig. 6. The SEM results of Sample C through immersion in HBSS
(Hanks’ balanced salt solution).
test. It indicated that this apatite layer was formed by ion exchange between BCP samples and HBSS. The formed apatite layer has been reported to increase the osteo-con- ductivity by promoting the adsorption of the osteo- blasts.
14-15)Thus, BCP samples fabricated in this study indicated good biological properties in HBSS.
The cell viabilities of the porous BCP samples and re- ference sample are shown(Fig. 7). Cell viabilities of BCP samples were improved with increasing SH contents. It causes high cell attachments of materials because of specific surface area increased by adding SH(Fig. 8).
Therefore, cell viabilities of the porous BCP biomaterials
increased by increasing SH contents.
4. Conclusions
From the experimental results, we summarized as follows;
1) The porous HA/ β-TCP biomaterials with the macro- pore size of 466-525 μm were successfully fabricated by adding NH
4HCO
3as space holder, MgO as binder and TiH
2as foaming agent by using rapid sintering of SPS.
2) The HA/ β-TCP(BCP) biomaterials fabricated by adding TiH
2have rough surface of pores.
3) Bio-activity of HA/ β-TCP biomaterials had excellent properties in HBSS.
4) Cell viabilities of BCP biomaterials were improved with increasing SH content.
Acknowledgement
This study was supported by the National Research Foundation(NRF) (No: 2013R1A1A4A01005459) of Korea.
The authors thanks for Professor Bung-Young Park of College of Veterinary Medicine in Chonbuk National University. He helps to analysis of biocompatibility of the porous BCP.
References
1. A. Amir, B. S. Abu, M. Norhamidi, S. Junaidi and I. R.
Mohd, Mater. Des., 55, 165 (2014).
2. L. L. Hench, J. Am. Ceram. Soc., 81, 1705 (1998).
3. K. Elayaraja, V. Sarath Chandra, M. I. Ahymah Joshy, R.
V. Sunganthi, K. Asokan and S. Narayana Kalkura, Appl.
Surf. Sci., 274, 203 (2013).
4. X. Yang, X. Lu, Q. Zhang, X. Zhang, Z. Gu and J. Chen,
Fig. 7. The MTT assay results of HA/β-TCP biomaterials.
Table 4. The ICP analysis of HBSS before immersion(B.I.) and after immersion(A.I.).
Sample Ca(ppm) P(ppm)
B.I. A.I. B.I. A.I.
HBSS(after 30 days) 25.132 9.109 11.786 3.610
Fig. 8. Cell attachments test results; (a), (b), (c) Sample A; (d), (e), (f) Sample D.
Mater. Sci. Eng. C, 27, 781 (2007).
5. D. M. Roy and S. K. Linnehan, Nature, 247, 220 (1974).
6. D. Tadic, F. Beckmann, K. Schwarz and M. Epple, Biomater., 25, 3335 (2004).
7. S. Aoki, S. Yamaguchi, A. Nakahira and K. Suganuma, J. Ceram. Soc. Jap., 112, 193 (2004).
8. S. H. Li, J. R. D. Wijin, P. Layrolle and K. D. Groot, J.
Am. Ceram. Soc., 86, 65 (2003).
9. S. M. Sharif, Z. A. Ahmad and M. R. Othman, Int. J.
Sci. Technol. Res., 2, 282 (2013).
10. P. Feng, M. Niu, C. Gao, S. Peng and C. Shuai, Sci.
Rep., 4, 5599 (2014).
11. Y. W. Gu, M. S. Yong, B. Y. Tay and C. S. Lim, Mater.
Sci. Eng. C, 29, 1515 (2009).
12. A. Arifin, A. B. Sulong, N. Muhamad, J. Syarif and M.
I. Ramli, Mater. Des., 55, 165 (2014).
13. L. Zhang and T. J. Webster, Nano Today, 4, 66 (2009).
14. R. Zhang and P. X. Ma, J. Biomed. Mater. Res., 45, 285 (1999).
15. S. Yamada, D. Heymann, J. M. Bouler and G. Daculsi, Biomater., 18, 1037 (1997).