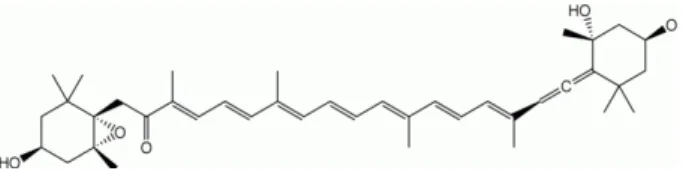
관련 문서
tricuspidata on the production of proinflammatory cytokines in TNFα+IFNγ-stimulated HaCaT cells ...15 Fig.5: The cell viability of sub-fractions from 70% EtOH
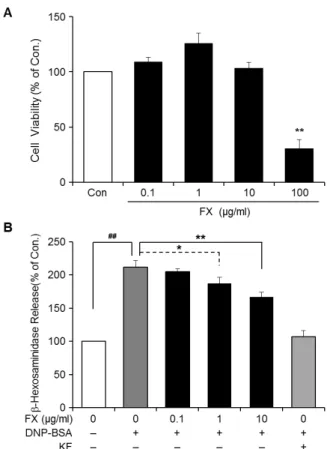
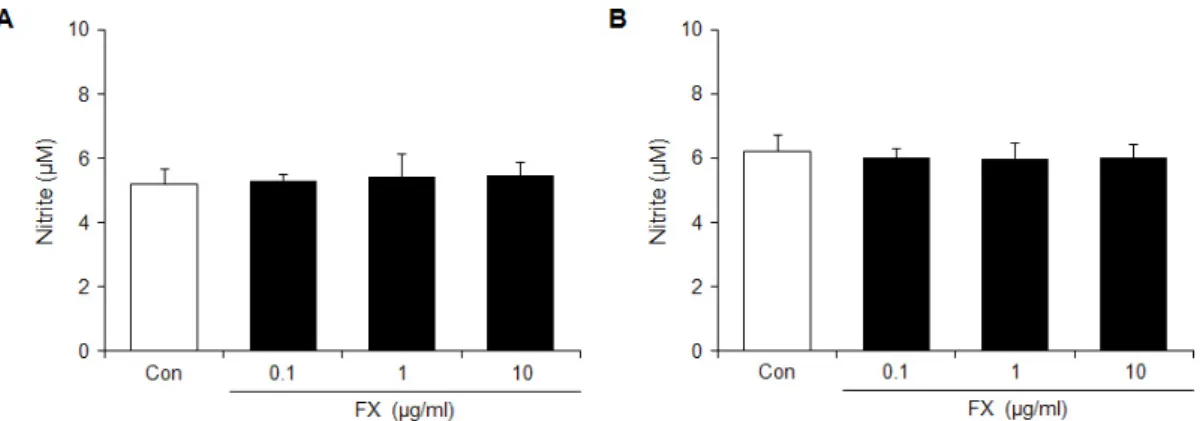
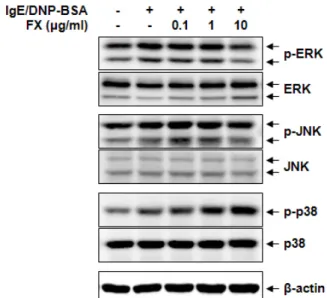
As for the research method, RBL-2H3 cells, a mast cell line, were treated with supercritical carbon dioxide extracted Chamaecyparis obtusa oil (C oil), German chamomile oil (G
The biological effect of ionizing radiation is a consequence of the energy transfer, by ionization and excitation, to cells in the body. Factors of radiation effects
This study supports that flavonoids present in PCE has anti-adipogenic and anti- diabetic effects by activating AMPK and normalizing the expression of
Effects of pulse frequency of low-level laser thrapy (LLLT)on bone nodule formation in rat calvarial cells.. Low-level laser therapy stimulats
Often found connected to other molecules on the outsides of cells --- cellular recognition, cell signaling, cell
• Generation of different specialized kinds of cells from zygote (fertilized egg) or other precursor cells.. – Generate blood cells, muscle
4 > Effects of Taro on COX-2 expression and iNOS expression(hot water) in human thyroid cancer cells. The cells were pretreated for 48hours with either