DOI 10.17480/psk.2017.61.5.241
Targeted Drug Delivery of Transferrin-Conjugated Mesoporous Silica Nanoparticles
Muhyun Jang and Injoon Oh
#College of Pharmacy, Chonnam National University, Gwangju 61186, Korea (Received August 28, 2017; Revised October 10, 2017; Accepted October 13, 2017)
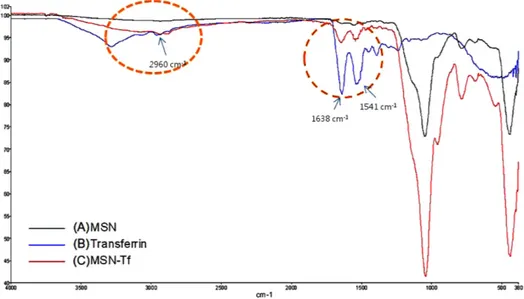
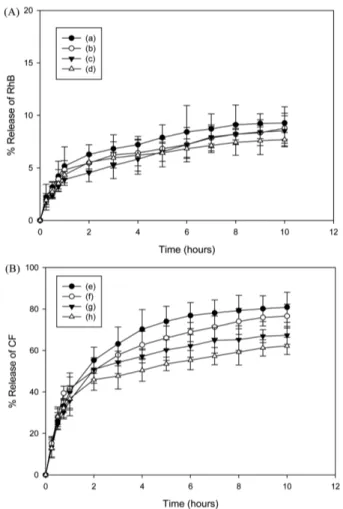
Abstract — The mesoporous silica nanoparticle (MSN) is a promising carrier as a drug-delivery system. And transferrin has been known as a ligand which binds to a receptor expressed on the surface of most proliferating cells such as tumor cells. In order to further improve the tumor delivery of MSN carrier, transferrin- conjugated MSNs were synthesized and evaluated as a targeting carrier. Fourier transform near infrared data revealed a conjugation of transferrin with MSN, while zeta potential and transmission electron microscopy results showed the nano-characteristics of the MSN in detail. To con- firm the optimal conditions for drug encapsulation onto the MSN, two types of dyes (hydrophilic rhodamine B and hydro- phobic 5,6-carboxyfluorescein) were loaded. Then, the differences in loading efficiencies and release properties of these dye- loaded MSNs were tested. The cellular uptake of dye-loaded MSNs was greater than dye solution. More importantly, the cellular uptake of transferrin modified MSNs was enhanced in the transferrin receptor-expressing Jurkat cells but not in nor- mal cells. Therefore, these results suggest that transferrin-modified MSN could be applied for therapeutic purposes as a drug-delivery system for targeting specific cancer cells.
Keywords Mesoporous silica nanoparticles, transferrin, targeted delivery, drug release, conjugation
Cancer is one of the most life threatening diseases that afflicts individuals all over the world. At present, various kinds of chemotherapeutic drugs have been developed. However, in many clinical cases, they often induce toxicity in normal tis- sues (such as loss of hair, changes in taste, and constant fatigue even under optimal conditions) due to their nonspecific actions. To overcome these side effects, there has been a lot of attention directed towards targeting the drug precisely and effi- ciently to the site of the cancer cell. In this study, the main strategy was to design a target-specific drug-delivery system that can transport an effective dose of the drug molecules to the precise cells and tissues.
1,2)The mesoporous silica nanoparticle (MSN) is a silica-based porous carrier that offers benefits such as a tunable particle size, stable and rigid framework, uniform pore size, high sur- face area and large pore volumes. Many studies have shown
that the MSN is biocompatible at the effective dose, and is capable of reducing the toxicity of anticancer drugs.
3,4)Nota- bly, MSN has an inner and an outer surface. This specialty allows the selective functionalization of the inner and outer surfaces of the MSN with different functional molecules like proteins or DNA.
5-7)MSN-based drug-delivery systems have also been reported to have the ability to bypass multidrug resistance mechanisms such as the P-glycoprotein efflux pump.
8)There have been several strategies taken to deliver chemo- therapeutic drugs to cancer cells more precisely. These include delivery approaches that avoids the reticuloendothelial sys- tem, utilizes the enhanced permeability and retention (EPR) effects, and are tumor-specific targeted.
9)Although EPR effects in themselves enable targeted delivery of MSN, devising outer surface functionalization of MSN-based particles with receptor- specific ligands (for example folic acid or IL-13) would further enhance the platform’s therapeutic profile by actively target- ing the pathology and simultaneously lessening the collateral damage.
10)Here, we used transferrin (Tf) protein as the tar- geting agent. The transferrin receptor (TfR) functions in the cellular uptake of iron through its interaction with Tf. This
#