JNK/FOXO-mediated Neuronal Expression of Fly Homologue
of Peroxiredoxin II Reduces Oxidative Stress and Extends
Life Span
*
□SReceived for publication, May 31, 2009, and in revised form, August 24, 2009 Published, JBC Papers in Press, August 31, 2009, DOI 10.1074/jbc.M109.028027
Kyu-Sun Lee‡, Kanae Iijima-Ando§1, Koichi Iijima¶1, Won-Jae Lee储**, Joon H. Lee‡‡, Kweon Yu‡2,
and Dong-Seok Lee§§3
From the‡Aging Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon 305-806, Korea, the §Laboratory of Neurogenetics and Pathobiology and¶Laboratory of Neurodegenerative and Metabolic Diseases, Farber Institute for Neurosciences, Department of Biochemistry and Molecular Biology, Thomas Jefferson University, Philadelphia,
Pennsylvania 19107, the储Department of Life Science, Ewha Woman’s University, and **National Creative Research Initiative Center for Symbiosystem, Seoul 120-750, Korea, the‡‡Myung-Gok Eye Research Institute, Kim’s Eye Hospital, College of Medicine, Konyang University, Chungnam 302-718, Korea, and the§§College of Natural Sciences, Kyungpook National University,
Daegu 702-701, Korea
Activation of c-Jun N-terminal kinase (JNK) signaling in neurons increases stress resistance and extends life span, in part through FOXO-mediated transcription in Drosophila. However, the JNK/ FOXO target genes are unknown. Here, we identified Jafrac1, a
Drosophila homolog of human Peroxiredoxin II (hPrxII), as a
downstream effecter of JNK/FOXO signaling in neurons that enhances stress resistance and extends life span. We found that
Jafrac1 was expressed in the adult brain and induced by
para-quat, a reactive oxygen species-generating chemical. RNA inter-ference-mediated neuronal knockdown of Jafrac1 enhanced, while neuronal overexpression of Jafrac1 and hPrxII sup-pressed, paraquat-induced lethality in flies. Neuronal expres-sion of Jafrac1 also significantly reduced ROS levels, restored mitochondrial function, and attenuated JNK activation caused by paraquat. Activation of JNK/FOXO signaling in neurons increased the Jafrac1 expression level under both normal and oxidative stressed conditions. Moreover, neuronal knockdown of Jafrac1 shortened, while overexpression of Jafrac1 and hPrxII extended, the life span in flies. These results support the hypoth-esis that JNK/FOXO signaling extends life span via amelioration of oxidative damage and mitochondrial dysfunction in neurons.
FOXO transcription factors are key regulators of growth, metabolism, life span, and stress resistance in various organ-isms, including Drosophila (1, 2). FOXO is regulated by the insulin signaling pathway and the stress-induced JNK4 signal-ing pathway (3, 4). Oxidative stress activates the stress-respon-sive JNK, which promotes FOXO nuclear localization and up-regulates expression of antioxidant proteins (5, 6).
In Drosophila, neuronal activation of JNK/FOXO signaling confers resistance to oxidative stress and extends life span (4, 7). Neurons are particularly susceptible to oxidative damage because of their high levels of ROS production and relatively low levels of antioxidant enzymes (8). Thus, activation of the JNK/FOXO pathway in neurons may extend life span through up-regulation of anti-oxidative stress genes. However, little is known regarding the JNK/FOXO target genes in neurons.
Thiol-reducing systems are important reducers of many oxi-dative stressors, such as peroxide (9). Peroxiredoxin (Prx), also called thioredoxin peroxidase, eliminates hydroperoxide with thioredoxin as an immediate hydrogen donor and reduces ROS levels (10). Among six distinct mammalian Prxs (I–VI), Prx II is exclusively expressed in the brain (11), suggesting that Prx II may play an important role in response to oxidative stress in neurons. However, the regulation of PrxII expression in neu-rons has not been elucidated. In this study, we demonstrated that neuronal expression of Jafrac1, a Drosophila homologue of human Prx II (hPrxII), was regulated by JNK/FOXO signaling, promoted resistance to oxidative stress, and extended the life span of the flies.
EXPERIMENTAL PROCEDURES
Drosophila Culture and Mutants—Drosophila melanogaster were kept at 25 °C and cultured using standard methods. Wild-type Oregon-R, w-, elav-Gal4 (pan-neuron driver),
Actin5C-Gal4 (ubiquitous driver), Cha-Gal4 (cholinergic neuron driver), repo-Gal4 (glial cell driver), elavGS-Gal4
(pan-neuro-*This work was supported, in whole or in part, by National Institutes of Health Grant R01AG032279 (to K. I. and K. I.-A.). This work was also supported by the Korea Science & Engineering Foundation (Grants 2009-0074555 and R01-2008-000-21076-0), by the BioGreen21 Program (Grant 20080401-034-050-008-01-00), by the Rural Development Administration, and by the Korea Research Institute of Bioscience and Biotechnology Research Initia-tive Program (Grant KGM3310912), Republic of Korea, by a pilot research grant from the Thomas Jefferson University (to K. I.), and by grants from the Gilbert Foundation/the American Federation for Aging Research (to K. I.) and the Alzheimer’s Association (Grant NIRG-08-91985 to K. I.).
□S The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Tables 1 and 2.
1Supported by start-up funds from the Farber Institute for Neurosciences. 2To whom correspondence may be addressed: 111 Gwahangno Yuseong-gu,
Daejeon 305-806, Korea. Tel.: 82-42-860-4642; Fax: 82-42-860-4608; E-mail: kweonyu@kribb.re.kr.
3To whom correspondence may be addressed: 1370 Sankyuk-dong, Buk-gu, Daegu 702-701, Korea. Tel.: 82-53-950-7366; Fax: 82-53-943-6925; E-mail: lee1@knu.ac.kr.
4The abbreviations used are: JNK, c-Jun NH
2-terminal kinase; JNKK, JNK kinase; Prx, peroxiredoxin; ROS, reactive oxygen species; PBS, phosphate-buffered saline; mtDNA, mitochondrial DNA; RNAi, RNA interference; pJNK, phospho-JNK.
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
Downloaded from
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
Downloaded from
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
Downloaded from
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
Downloaded from
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
Downloaded from
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
nal GeneSwitch driver), and UAS-dFOXO.WT flies were obtained from the Bloomington Stock Center. Jafrac1G1104
mutants, which carries an EP element (12) inserted at 5 ⬘-un-translated region of the Jafrac1, were purchased from GenExel, Inc. (Daejeon, Korea). The JNK signaling related bsk1/CyO
(Amino acid replacement: G225E), hep1/FM6(mutant carries a
P element inserted at a position 179 nucleotides upstream of the ATG codon), and UAS-hepCA mutants (amino acid
resi-dues, Ser-346, Thr-350, and Ser-352 in Hep were all replaced with Asp) were gifts from J. Chung. The UAS-dFOXO.TM (a triple PKB phosphorylation sites mutant of dFOXO: T44A, S190A, and S259A), dFOXO21/TM6B, and dFOXO25/TM6B
lines were gifts from E. Hafen and K. J. Min. In dFOXO21and
dFOXO25, the codons for Trp-95 and Trp-124 within the
fork-head domain are mutated to stop codons, respectively (13), so they are assumed to be null alleles of dFOXO. The Drosophila
Jafrac1lines (UAS-Jafrac1 and UAS-Jafrac1-RNAi) were previ-ously described in Ha et al. (14).
Paraquat Treatment—To investigate the effect of oxidative stress in the Drosophila model, adult flies (5 days old) were exposed in 20 mMparaquat for 24 h. The flies were kept in vials containing 1 ml of 1.3% agar for 6 h for starvation before the paraquat treatment. The flies were then transferred to vials containing a 22-mm filter paper disk soaked with 20 mM para-quat (methyl viologen, Sigma) in 5% sucrose solution. The data are presented as means⫾ S.E. The statistically significant dif-ferences were examined using the Student’s t test (Microsoft Excel) and p⬍ 0.05 was accepted as statistically significant.
Measuring ROS Levels in Drosophila—To measure the inter-cellular ROS level in Drosophila, we used the method described by Strayer et al. (15). Non-fluorescent 2,7-dichlorofluorescein di-acetate (Molecular Probes) is a cell permeable dye and can be converted into 2,7-dichlorofluoroscein by interacting with hydrogen peroxide (16). Flies (3 days old) were treated with 20 mMparaquat for 24 h and collected in tubes containing 500l of PBST (PBS containing 0.1% Tween 20). The flies were then homogenized, and 100l of each supernatant was transferred into a 96-well plate. After adding 50M2,7-dichlorofluorescein diacetate to the samples, the fluorescence intensity was meas-ured every 5 min for 15 min using a fluorescence microplate reader (FLUOstar Optima, BMG Laboratory) and the fluores-cence intensity (excitation 485 nm and emission 640 nm) was quantified. Three independent experiments with 50 flies in each experiment were performed.
ATP Assay and mtDNA PCR Analysis—Total ATP produc-tion was measured as previously described (17). Heads of 10 2-day-old flies were dissected and homogenized in extraction buffer (100 mM Tris and 4 mM EDTA, pH 7.8) followed by quick-freezing in liquid nitrogen and boiling for 3 min. The samples were then centrifuged to collect the supernatant and mixed with luminescent solution (Enliten kit, Promega, Madi-son, WI). The luminescence was measured using a luminome-ter (FLUOstar Optima, BMG Laboratory), and the results were compared with standards. The ATP level was measured relative to total protein concentration. For mtDNA PCR, total DNA was extracted from 2-day-old flies and subjected to PCR ampli-fication with primer sets for various target genes (supplemental Table 1). The genomic DNA level of rp49 of each sample was
used as the loading control. Results are expressed as -fold change relative to control.
Semi-quantitative Reverse Transcription-PCR Analysis—For reverse transcription-PCR analysis, 1g of total RNA was used with the oligo(dT) primer and avian myeloblastosis virus reverse transcriptase (Roche Biochemical) to generate first strand cDNA. Next, 1l of the cDNA was subjected to PCR amplification with the primer sets for various target genes ( sup-plemental Table 1). PCR conditions were 94 °C for 5 min, fol-lowed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 10 min using a Thermal Cycler (Applied Bioscience). PCR products were resolved in 1.5% agarose gels and visualized by ethidium bromide staining. The rp49 gene was used as a control.
Protein Analysis and Immunostaining—Western blot analy-ses were performed as described previously (18). The antibod-ies against JNK (Santa Cruz Biotechnology, Santa Cruz, CA) and phospho-JNK (Promega) were used to detect JNK activa-tion. The antibody against dFOXO (a gift from O. Puig) was used to detect FOXO translocation to the nucleus. To detect pJNK and FOXO in vivo, the third instar larvae were fed with 20 mMparaquat for 24 h, and then cuticles were dissected and fixed in 4% paraformaldehyde in PBS for 1 h at room tempera-ture and incubated with the pJNK antibody (1:200) or FOXO antibody (1:500) and, subsequently, with Alexa Fluor 594-con-jugated anti-rabbit IgG (1:200, Molecular Probes). Fluores-cence images were acquired using an Axiovert 200M micro-scope (Carl Zeiss, Germany).
Life Span Assay—For longevity experiments, 1- and 2-day-old adult male or female flies were collected (10 per vial), trans-ferred to fresh medium every 2 days, and scored for survivors. The starting population for each genotype was 100 flies. Three replicates were tested for each genotype. Survival, lx, was esti-mated as Nx/N0, where Nxis the number of flies alive at the beginning of each census interval, and N0is the initial cohort size. Significant differences in survival between pairs of cohorts were tested using the log-rank test.
RESULTS
Drosophila Jafrac1 Is an Ortholog of Human PrxII—Based on amino acid sequence similarity, we identified a Drosophila per-oxiredoxin IIgene (Jafrac1, CG1633, Dpx-4783) that belongs to the 2-Cys peroxiredoxin subfamily. The amino acid sequence of the Jafrac1 protein shows significant homology to hPrxII, including conserved cysteine motifs (Fig. 1A), and the overall amino acid similarity of 83% between Jafrac1 and hPrxII (157/ 188). There are six Drosophila homologs of human peroxire-doxins that share at least 60% amino acid identity. Among these, the molecular characters of Jafrac1 are most similar with hPrxII, including protein size, the number of conserved cys-teine residues, and subcellular localization (supplemental Table 2). Jafrac1 mRNA is expressed throughout development, and in the third instar larvae, Jafrac1 mRNA is abundant in brain, imaginal discs, and Malpighian tubules (supplemental Fig. S1). Neuronal Expression of Jafrac1 or hPrxII ROS-induced Lethality—The expression of Jafrac1 was induced in wild-type flies treated with 20 mMparaquat for 24 h (Fig. 1B). To test the effect of Jafrac1 overexpression on oxidative stress,
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
we generated transgenic fly lines carrying the Jafrac1 or hPrxII genes under the control of the UAS promoter. Reverse transcrip-tion-PCR (Jafrac1) and Western blot analysis (hPrxII) confirmed that both lines exhibited Gal4-dependent up-regulation of Jafrac1 transcripts and hPrxII proteins, respectively (supplemental Fig. S2). Ubiquitous expression of Jafrac1 using Actin5C-Gal4 demonstrated that Jafrac1 expression reduced oxi-dative stress-induced lethality (Fig. 1C). We next tested whether neuro-nal overexpression of Jafrac1 is suf-ficient to confer resistance to para-quat treatment. Using elav-Gal4, which drives expression in all post-mitotic neurons (19), and Cha-Gal4, which drives expression pri-marily in cholinergic neurons (20), we found that elav⬎Jafrac1 and Cha⬎Jafrac1 adult flies exhibited significantly reduced paraquat-in-duced lethality, comparable to Actin5C⬎Jafrac1 flies (Fig. 1C). Interestingly, Jafrac1 overexpres-sion in glial cells using repo-Gal4 was not protective. In contrast, neuronal knockdown of Jafrac1, but not knockdown of Jafrac1 in glial cells, sensitized flies to paraquat-induced lethality (data not shown). These results suggest that Jafrac1 plays a protective role against oxidative stress in neurons.
Importantly, ROS hypersensi-tivity of RNAi-mediated knock-down of Jafrac1 in flies was res-cued by overexpression of human PrxII (elav⬎Jafrac1-Ri;hPrxII) (Fig. 1C), suggesting that human PrxII is a functional homolog of Dro-sophila Jafrac1.
To test the role of Jafrac1 in ROS metabolism, we measured ROS levels using a 2,7-dichlorofluores-cein-DA dependent fluorescence
FIGURE 1. Neuronal expression of Jafrac1, a Drosophila ortholog of hPrxII, enhances resistance to oxidative stress. A, amino acid sequence alignments of PrxII and Jafrac1 show amino acid homologies (dark backgrounds) and conserved cysteine residues (boxes). B, expression of Jafrac1 in flies treated with 20 mM paraquat treatment for 24 h. rp49 mRNA was used as a control. C, paraquat-induced lethality of flies overexpressing Jafrac1 or with Jafrac1 knockdown driven by tissue-specific enhancers. Neuronal overexpression of hPrxII rescued the increased lethality by elav⬎Jafrac1-RNAi (elav⬎Jafrac1-RNAi; hPrxII). Each bar represents the mean⫾ S.E. from four independent experiments (n ⫽ 200 per experiment, *, p ⬍ 0.05, Student’s t test). D, ROS levels after paraquat treatment. Neuronal overexpression of Jafrac1 (elav⬎Jafrac1) or hPrxII (elav⬎ hPrxII) reduced the ROS level, whereas inhibition of Jafrac1 expression in neurons (elav⬎Jafrac1-RNAi) increased the ROS level compared with the wild-type control. Mean ⫾ S.E. from three independent experiments (n ⫽ 50 per each experiment; *, p⬍ 0.05; **, p ⬍ 0.001; Student’s t test).
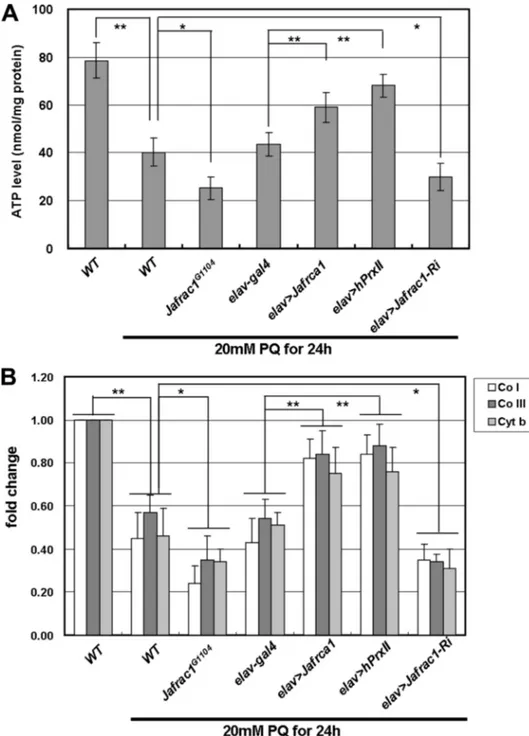
FIGURE 2. Neuronal expression of Jafrac1 or hPrxII restores oxidative stress-induced mitochondrial dys-function. ATP levels in flies exposed to 20 mMparaquat. Flies with neuronal overexpression of Jafrac1 (elav⬎Jafrac1) or hPrxII (elav⬎ hPrxII) exhibit higher ATP levels compared with wild-type controls (WT), whereas ATP levels were reduced in the Jafrac1G1104mutant and neuronal Jafrac1 inhibition (elav⬎Jafrac1-RNAi) flies.
Data are expressed as mean⫾ S.E. from four independent experiments (n ⫽ 10 per each experiment; *, p ⬍ 0.05; **, p⬍ 0.001; Student’s t test). Copy numbers of mitochondrial marker genes (Co I: cytochrome c oxidase subunit I; Co III: cytochrome c oxidase subunit III; and Cyt C: cytochrome c) in the flies after paraquat treatment. The copy number reduction was significantly aggravated in the Jafrac1G1104mutant and neuronal Jafrac1
inhibition (elav⬎Jafrac1-RNAi) flies and restored by neuronal overexpression of Jafrac1 or hPrxII (elav⬎Jafrac1 or elav⬎hPxII). Mean ⫾ S.E. from three independent experiments (n ⫽ 5 per each experiment; *, p ⬍ 0.05; **,
p⬍ 0.001; Student’s t test).
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
assay. When wild-type flies were exposed to 20 mMparaquat, inter-cellular ROS levels were signifi-cantly increased in a time-depend-ent manner: 3-fold and 3.5-fold after 6- and 12-h exposures, respec-tively. The intracellular ROS levels were dramatically reduced by neu-ronal overexpression of Jafrac1 or
hPrxII(elav⬎Jafrac1 or elav⬎hPrxII) (Fig. 1D). Conversely, flies with neuronal knockdown of Jafrac1 (elav⬎Jafrac1-Ri) showed in-creased ROS levels compared with wild-type control flies (Fig. 1D). These results indicate that both Jafrac1 and hPrxII can mod-ulate intracellular ROS levels in
Drosophila.
Neuronal Expression of Jafrac1 or hPrxII ROS-induced Mitochondrial Dysfunction—To test whether Jafrac1 has a protective effect on mitochon-drial function under oxidative stress, we measured ATP levels in flies with neuronal overexpression of Jafrac1 or hPrxII after paraquat treatment. In the control flies, 20 mM paraquat treatment resulted in a 50% reduction in the ATP level. Neuronal overexpression of Jafrac1 or hPrxII (elav⬎Jafrac1, elav⬎hPrxII) mark-edly restored ATP production, whereas the reduction of ATP lev-els after paraquat treatment was en-hanced in loss-of-function Jafrac1 mutants (Jafrac1G1104) and flies with neuronal knockdown of Jafrac1 (elav⬎Jafrac1-Ri) (Fig. 2A).
We next quantified mitochondrial abundance by measuring the mito-chondrial DNA (mtDNA) copy num-ber. Treatment with 20 mMparaquat caused a marked reduction in the levels of mtDNA, and this reduction in mtDNA levels induced by para-quat treatment was restored by
Jafrac1or hPrxII overexpression in neurons (Fig. 2B). The reduction in mtDNA levels after paraquat treat-ment was enhanced in loss-of-func-tion Jafrac1 mutants (Jafrac1G1104)
and flies with neuronal knockdown of Jafrac1 (elav⬎Jafrac1-Ri). These data indicate that neuronal expres-sion of Jafrac1 or hPrxII restores oxidative stress-induced mitochon-drial function.
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
Neuronal Expression of Jafrac1 Inhibits Oxidative Stress-in-duced JNK Activation—Oxidative stress can activate the stress-responsive JNK/FOXO signaling pathway (3, 4, 6, 7). To exam-ine JNK and FOXO activation by oxidative stress in cholexam-inergic neurons, we marked cholinergic neurons with green fluores-cent protein (Cha⬎GFP) and immunostained with the
phos-pho-JNK (pJNK) and FOXO anti-body to detect the active form of JNK and FOXO, respectively, in the neuromuscular junctions of third instar larvae. Activated JNK was observed in the cholinergic neurons treated with 20 mMparaquat (Fig. 3B, yellow), but not in the controls (Fig. 3A). Similarly, FOXO protein was localized in the nuclei of cholin-ergic neurons treated with 20 mM paraquat (Fig. 3D, arrow), but not in the controls (Fig. 3C).
Jafrac1expression (Cha⬎ Jafrac1) reduced the number of pJNK-positive neurons (Fig. 3E). Western blot anal-ysis following treatment with 20 mM paraquat revealed that the treatment induced activation of JNK, but over-expression of Jafrac1 in neurons (elav⬎Jafrac1 and Cha⬎Jafrac1) suppressed JNK activation (Fig. 3F). These results suggest that oxida-tive stress-induced JNK activation can be suppressed by Jafrac1 in neurons.
Peroxiredoxin (Prx) enzymes mod-ulate oxidative stress via its evolution-ary conserved cysteine (Cys) residues (21). Under oxidative stress condi-tion, cysteine sulfinic acid of Prx is oxidized. To test whether a
Dro-sophilaPrx homolog, Jafrac1, is also oxidized under oxidative stress con-dition, we performed Western blot analysis with oxidized Prx specific antibody, anti-Prx-SO3. In wild-type flies, the level of oxidized Prx homolog was increased after 20 mM paraquat for 24 h, indicating that the oxidation of Cys residue is con-served in Jafrac1. Increase of oxida-tion of Jafrac1 was enhanced in a hemizygous mutation of JNKK (Hep1/Y) or transallelic mutation of dFOXO21/25compared
the level of wild-type control flies after paraquat treatment, pre-sumably by increased ROS levels in these mutant backgrounds (Fig. 3G). These results further support that Jafrac1 modulates oxi-dative stress in Drosophila.
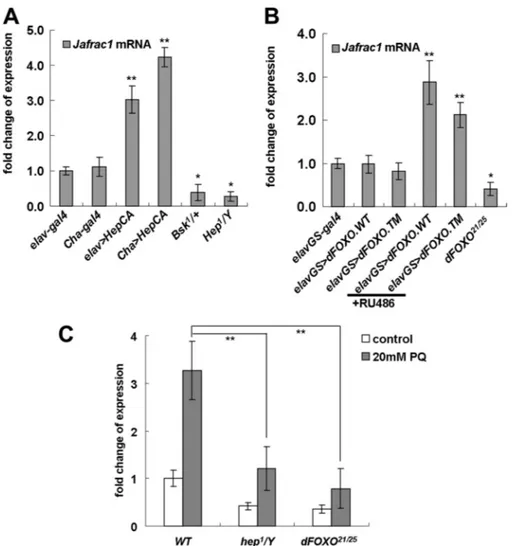
FIGURE 3. Neuronal expression of Jafrac1 inhibits oxidative stress-induced JNK activation. Cha⬎GFP marked cholinergic neurons in Drosophila third instar larvae (green) and 4⬘,6-diamidino-2-phenylindole (DAPI)-stained nuclear DNA in muscle (blue). A and B, activated JNK (p-JNK) in cholinergic neurons after paraquat treatment (yellow, white arrows). C and D, nuclear translocation of FOXO transcription factors after paraquat treatment in cholinergic neurons (arrows). E, number of pJNK-positive neurons after paraquat treatment. 60% of cholinergic neurons were pJNK-positive in the wild type compared with 25% in flies overexpressing Jafrac1 (Cha⬎Jafrac1). Mean ⫾ S.E. from five independent samples (*, p ⬍ 0.05; **, p ⬍ 0.001; Student’s t test). F, Western blotting with anti-pJNK (top) and anti-JNK (middle). A representative blot and the quantification of signals as the ratio of pJNK and JNK (mean⫾ S.E., n ⫽ 3) are shown (bottom). Neuronal overexpression of Jafrac1 (elav⬎Jafrac1 and Cha⬎Jafrac1) suppressed the oxidative stress-induced phosphorylation of JNK. G and H, oxidation of peroxiredoxin in wild-type or JNK/FOXO mutants under oxidative stress condition. G, Western blot analysis with oxidized peroxiredoxin-specific antibody, anti-Prx-SO3. H, Coomassie Blue staining image of transferred gel used in Western blot analysis confirms equal protein loading.
FIGURE 4. Jafrac1 expression is regulated by the JNK/FOXO signaling pathway. mRNA levels of Jafrac1 in flies with genetic manipulations of the JNK pathway. A, overexpression of the constitutively active form of JNKK in neurons (elav⬎HepCA), or in cholinergic neurons (Cha⬎HepCA), increased levels of Jafrac1 mRNA, and heterozygous mutants of JNK (Bsk1/⫹) or a hemizygous mutation of JNKK (Hep1/Y) decreased the Jafrac1 mRNA
level, compared with controls (elav-Gal4 and Cha-Gal4). B, mRNA levels of Jafrac1 in flies with genetic manip-ulations of FOXO. Expression of wild-type dFOXO or the constitutively active form of dFOXO (dFOXO TM) was induced in adult flies using the inducible neuronal gene switch driver (elavGS) by RU486 treatment. Neuronal overexpression of dFOXO (elavGS⬎dFOXO WT, ⫹RU486) and dFOXOTM (elavGS⬎dFOXO TM⫹RU486) increased expression levels of Jafrac1 compared with flies carrying driver-only constructs or flies not treated with RU486 (elavGS-Gal4, elavGS⬎dFOXO WT, or elavGS⬎dFOXO TM). In contrast, the dFOXO21/25mutation
decreased the expression of Jafrac1. C, expression of Jafrac1 in JNK/FOXO mutant flies treated with 20 mM paraquat for 24 h. Expression of Jafrac1 is increased in wild-type control flies exposed to oxidative stress, but this increase was significantly reduced in a hemizygous mutation of JNKK (Hep1/Y) or transallelic dFOXO21/25
mutation. Mean⫾ S.E. from three independent experiments (n ⫽ 10 per each experiment; *, p ⬍ 0.05; **, p ⬍ 0.001; Student’s t test).
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
JNK/FOXO Signaling Regulates Jafrac1 Expression under Both Normal and Oxidative Stressed Conditions—JNK/FOXO signaling activates expression of several genes involved in cel-lular stress responses (4). To test whether JNK/FOXO signaling regulates Jafrac1 expression, we first examined the effect of genetic manipulation of JNK activity in neurons on Jafrac1 gene expression. Neuronal overexpression of the constitutively active form of hemipterus (hep), a Drosophila JNKK gene (elav⬎hepCAand cha⬎hepCA), markedly increased the
expres-sion level of Jafrac1. In contrast, Jafrac1 mRNA levels are reduced in flies carrying one copy of a loss of function mutation of the Drosophila homolog of JNK, Basket (Bsk1), in the
hem-izygous hep1mutant (hep1/Y) background (Fig. 4A).
Next, we tested the effect of genetic manipulation of FOXO on Jafrac1 gene expression. To induce FOXO expression in neu-rons in the adult stage, we used the RU486-inducible elav-Gal4 driver (elavGS-Gal4). Neuronal overex-pression of wild-type FOXO
(elavGS⬎dFOXO ⫹RU) or the insulin-insensitive nuclear form of
FOXO(elavGS⬎dFOXO.TM ⫹RU) increased the expression level of
Jafrac1by ⬎2-fold compared with the controls, whereas the expres-sion of Jafrac1 was reduced in a
FOXOmutant (Foxo21/25) (Fig. 4B).
In addition, the expression of
Jafrac1was over 3-fold increased in wild-type control flies treated with 20 mMparaquat for 24 h, but this increase was significantly reduced in a hemizygous mutation of JNKK (Hep1/Y) or transallelic mutation
of dFOXO21/25 (Fig. 4C). These
results indicate that JNK/FOXO signaling regulates Jafac1 expres-sion in neurons.
Neuronal Expression of Jafrac1 or hPrxII Extends Life Span in Flies— Because it is well established that activation of JNK/FOXO signaling increases life span (2, 4, 7), we exam-ined the role of neuronal Jafrac1, a target gene of JNK/FOXO signaling, in the control of the fly life span. Neuronal overexpression of Jafrac1 or hPrxII in neurons (elav⬎Jafrac1 or elav⬎hPrxII) significantly in-creased life span, while neuronal knockdown of Jafrac1 (elav
⬎Jafrac1-Ri), as well as the loss-of-function mutation (Jafrac1G1104), caused a
reduction in life span (Fig. 5A). To test whether neuronal expression of
Jafrac1 in the adult stage is suffi-cient to extend life span, we used the RU486-inducible
elav-Gal4driver (elavGS-Gal4) to express Jafrac1 in adult neurons (22). It has been reported that RU486 feeding does not affect life span of flies (2). Expression of Jafrac1 in adult neurons extended life span by 26% in females (Fig. 5B) and 29% in males (Fig. 5C), compared with the control flies.
DISCUSSION
Multiple lines of evidence point to the activation of the JNK/ FOXO pathway as a common cellular response to oxidative damage across animal phyla (4, 6, 7, 13, 23). In Drosophila, JNK confers tolerance to oxidative stress and extends life span by inducing a protective gene expression program. Increased JNK
FIGURE 5. Neuronal expression of Jafrac1- or hPrxII-extended life span. A, neuronal overexpression of
Jafrac1 or hPrxII (elav⬎Jafrac1 or elav⬎hPrxII) extended the life span of the flies, whereas the Jafrac1G1104
mutant and neuronal knockdown of Jafrac1 (elav⬎Jafrac1-RNAi) reduced the life span compared with the wild-type and elav-Gal4 controls (n⫽ 200 per each genotype). B and C, extended life span in flies with neuronal overexpression of Jafrac1 induced in the adult stage using the GeneSwitch driver (elavGS-Gal4). Male and female flies with Jafrac1 expression in adult neurons induced by RU486 show increased average, median, and maximum life spans compared with untreated control flies (log rank test, male,2⫽ 1.50, p ⬍ 0.0001, n ⫽ 189;
female,2⫽ 0.91, p ⬍ 0.0001, n ⫽ 197). D, a model of the JNX/FOXO pathway. Oxidative stress by the paraquat
treatment induces mitochondrial damage and activates the JNK/FOXO pathway. This stress induces neuronal expression of Jafrac1, which in turn suppresses oxidative stress-induced lethality and extends life span.
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
activity in neurons is sufficient to promote stress tolerance and extend life span in flies (4, 7). However, whether this effect is due to the specific protection of neurons against oxidative dam-age, or whether JNK activation in neurons may induce a humoral response that regulates longevity systemically, is unclear (1, 5).
In this study, we demonstrated that JNK/FOXO signaling is required for the expression of Jafrac1 in brains under both nor-mal and oxidative stressed conditions (Fig. 4). There are two putative FOXO consensus binding sites (RWWAACA) in the promoter region of Jafrac1 (data not shown), suggesting that the transcription factor FOXO may bind to the Jafrac1 pro-moter and directly activate Jafrac1 transcription. We also dem-onstrated that neuronal knockdown of Jafrac1 enhances, and neuronal overexpression of Jafrac1 reduces, ROS-induced lethality. Furthermore, the neuronal knockdown of Jafrac1 shortened, while overexpression of Jafrac1 extended, the life span of the flies. These results support the hypothesis that, in
Drosophila, the JNK/FOXO pathway protects neurons from oxidative stress and extends life span by induction of antioxi-dant genes, including Jafrac1 in neurons (Fig. 5D).
Peroxiredoxins (Prxs) are identified by their ability to neu-tralize cellular hydroperoxides in mammals (24). A family of five Prx genes has been identified and characterized in D.
mela-nogaster(25). All Drosophila Prxs have peroxidase activities, and their expressions are induced by oxidative stress. Prx over-expression enhances resistance to oxidative stress by hydrogen peroxide and paraquat in cultured Drosophila cells (25, 26). In
Drosophila, overexpression of Jafrac1 has been shown to coun-teract the enhanced susceptibility of immune-regulated cata-lase knockdown flies to natural infections (14). Moreover, mito-chondrial peroxiredoxin (Dpx-5037, mTPx) has been reported to restore wild-type life span in a Drosophila model for Fried-reich’s ataxia (27).
We have demonstrated that neuronal expression of Jafrac1 and hPrxII significantly reduces the ROS level and restores mitochondrial function in paraquat-treated flies (Figs. 1D and 2). Several studies in Drosophila show that expression of spe-cific mitochondrial proteins can increase resistance to oxida-tive stress as well as extend life span (28 –30), suggesting that mitochondrial function plays an important role in determining life span. Collectively, Jafrac1 or hPrxII may extend life span by acting as a guardian for neuronal mitochondria under age-as-sociated oxidative stress conditions. Furthermore, because mitochondrial dysfunction is associated with many neurode-generative diseases (8), induction of Jafrac1/PrxII in neurons may also be protective against age-associated neurodegenera-tive diseases.
Acknowledgments—We thank J. Chung, E. Hafen, O. Puig, and K. J. Min for fly stocks and antibody.
REFERENCES
1. Accili, D., and Arden, K. C. (2004) Cell 117, 421– 426
2. Hwangbo, D. S., Gershman, B., Tu, M. P., Palmer, M., and Tatar, M. (2004)
Nature 429,562–566
3. Essers, M. A., Weijzen, S., de Vries-Smits, A. M., Saarloos, I., de Ruiter, N. D., Bos, J. L., and Burgering, B. M. (2004) EMBO J. 23, 4802– 4812 4. Wang, M. C., Bohmann, D., and Jasper, H. (2005) Cell 121, 115–125 5. Huang, H., and Tindall, D. J. (2007) J. Cell Sci. 120, 2479 –2487 6. Kops, G. J., Dansen, T. B., Polderman, P. E., Saarloos, I., Wirtz, K. W.,
Coffer, P. J., Huang, T. T., Bos, J. L., Medema, R. H., and Burgering, B. M. (2002) Nature 419, 316 –321
7. Wang, M. C., Bohmann, D., and Jasper, H. (2003) Dev. Cell 5, 811– 816 8. Lin, M. T., and Beal, M. F. (2006) Nature 443, 787–795
9. Matsumoto, A., Okado, A., Fujii, T., Fujii, J., Egashira, M., Niikawa, N., and Taniguchi, N. (1999) FEBS Lett. 443, 246 –250
10. Lim, Y. S., Cha, M. K., Kim, H. K., Uhm, T. B., Park, J. W., Kim, K., and Kim, I. H. (1993) Biochem. Biophys. Res. Commun. 192, 273–280
11. Jin, M. H., Lee, Y. H., Kim, J. M., Sun, H. N., Moon, E. Y., Shong, M. H., Kim, S. U., Lee, S. H., Lee, T. H., Yu, D. Y., and Lee, D. S. (2005) Neurosci.
Lett. 381,252–257
12. Rørth, P. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 12418 –12422
13. Ju¨nger, M. A., Rintelen, F., Stocker, H., Wasserman, J. D., Ve´gh, M., Rad-imerski, T., Greenberg, M. E., and Hafen, E. (2003) J. Biol. 2, 20 14. Ha, E. M., Oh, C. T., Ryu, J. H., Bae, Y. S., Kang, S. W., Jang, I. H., Brey, P. T.,
and Lee, W. J. (2005) Dev. Cell 8, 125–132
15. Strayer, A., Wu, Z., Christen, Y., Link, C. D., and Luo, Y. (2003) FASEB J. 17,2305–2307
16. Royall, J. A., and Ischiropoulos, H. (1993) Arch. Biochem. Biophys. 302, 348 –355
17. Park, J., Lee, S. B., Lee, S., Kim, Y., Song, S., Kim, S., Bae, E., Kim, J., Shong, M., Kim, J. M., and Chung, J. (2006) Nature 441, 1157–1161
18. Lee, K. S., You, K. H., Choo, J. K., Han, Y. M., and Yu, K. (2004) J. Biol.
Chem. 279,50781–50789
19. Lin, D. M., and Goodman, C. S. (1994) Neuron 13, 507–523
20. Salvaterra, P. M., and Kitamoto, T. (2001) Brain Res. Gene Expr. Patterns 1, 73– 82
21. Woo, H. A., Chae, H. Z., Hwang, S. C., Yang, K. S., Kang, S. W., Kim, K., and Rhee, S. G. (2003) Science 300, 653– 656
22. Osterwalder, T., Yoon, K. S., White, B. H., and Keshishian, H. (2001) Proc.
Natl. Acad. Sci. U.S.A. 98,12596 –12601
23. Tatar, M., Bartke, A., and Antebi, A. (2003) Science 299, 1346 –1351 24. Seaver, L. C., and Imlay, J. A. (2001) J. Bacteriol. 183, 7173–7181 25. Radyuk, S. N., Klichko, V. I., Spinola, B., Sohal, R. S., and Orr, W. C. (2001)
Free Radic Biol. Med. 31,1090 –1100
26. Radyuk, S. N., Sohal, R. S., and Orr, W. C. (2003) Biochem. J. 371, 743–752 27. Anderson, P. R., Kirby, K., Orr, W. C., Hilliker, A. J., and Phillips, J. P.
(2008) Proc. Natl. Acad. Sci. U.S.A. 105, 611– 616
28. Fridell, Y. W., Sa´nchez-Blanco, A., Silvia, B. A., and Helfand, S. L. (2005)
Cell Metab. 1,145–152
29. Morrow, G., Samson, M., Michaud, S., and Tanguay, R. M. (2004) FASEB
J. 18,598 –599
30. Mourikis, P., Hurlbut, G. D., and Artavanis-Tsakonas, S. (2006) Proc. Natl.
Acad. Sci. U.S.A. 103,1307–1312
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/
Supplementary Table 1 PCR Primers used in this study.
PCR primer
product size
sequences
Jafrac1
a188 bp
forward : CCCGAAAACTTTTAGACTCAreverse : TTTTCAAACATTTCCATCGT
Jafrac1
580 bp
forward : ATGCCCCAGCTACAGAAGCCreverse : TTAGGAGGTGGTCTCGAAGTCOX I
a157 bp
forward : GGTGCTCCTGATATAGCATTCCCreverse : CACCATGAGCAATTCCAGCG
COX III
a127 bp
forward : TTTTATAGCAACAGGATTCCACGGreverse : TGCAGCTGCTTCAAAACCAAA
Cytochrome b
a166 bp
forward : CACCTGCCCATATTCAACCAGAreverse : GGATAAAATTGAATCCCTCGGAA
rp49
206 bp
forward : AGATCGTGAAGAAGCGCACCAAG reverse : CACCAGGAACTTCTTGAATCCGGrp49
a122 bp
forward : AGGGTATCGACAACAGAGTGreverse : CACCAGGAACTTCTTGAATC
Note :
aprimers for the quantitative RT-PCR
Supplementary Table 2 The molecular characters of the Drosophila peroxiredoxin family.
References : Radyuk et al., 2001. Free Radical Biol & Med.; Radyuk et al, 2009. Bichemical J.
Gene name(amino acids) CG No.
Mammalian homolog
(amino acids) subgroup
Subcellular localization
Jafrac1 (194)
Dpx-4783 CG1633
PrxII (198)
PrxI (199) 2 cys Prx Cytosolic
Jafrac2 (242)
Dpx-4156 CG1274 PrxIV(271) 2 cys Prx Secretable
Dpx-2540 (220) CG12405
CG11765 PrxVI (224) 1 cys Prx Cytosolic
Dpx-5037 (234) CG5826 PrxIII (256) 2 cys Prx Mitochondrial
Dpx-6005 (388) CG3083 PrxVI (224) 1 cys Prx Cytosolic
E(0-4) E(4-24) 1L 2L 3L P M F
Jafrac1
rp49
Br Sal Fat Disc Hm Gut Mal
Jafrac1
rp49
A
B
Supplementary Fig.S1. Pattern of Jafrac1 expression during developmental stages (A) and in various
EP(X)G1104
Jafrac1
rp49
elav-gal4
elav>jafrac1
Jafrac1
G11
0
4
WT
Actin5C>hPrxII
hPrxII
β-actin
elav>hPrxII
B
C
A
Supplementary Fig.S2. Jafrac1 expression level in mutant flies. (A) The EP(X)G1104 element was
inserted in the Jafrac1 locus. (B) RT-PCR analysis of Jafrac1 mutants. (C) Western blot analysis of
hPrxII mutants.
and Dong-Seok Lee
Kyu-Sun Lee, Kanae Iijima-Ando, Koichi Iijima, Won-Jae Lee, Joon H. Lee, Kweon Yu
Reduces Oxidative Stress and Extends Life Span
doi: 10.1074/jbc.M109.028027 originally published online August 31, 2009 2009, 284:29454-29461.
J. Biol. Chem.
10.1074/jbc.M109.028027 Access the most updated version of this article at doi:
Alerts:
When a correction for this article is posted •
When this article is cited •
to choose from all of JBC's e-mail alerts Click here
Supplemental material:
http://www.jbc.org/content/suppl/2009/08/31/M109.028027.DC1.html http://www.jbc.org/content/284/43/29454.full.html#ref-list-1This article cites 30 references, 12 of which can be accessed free at
at Ewha Medical Library on October 24, 2016
http://www.jbc.org/