International Journal of
Molecular Sciences
Article
6-Pyrazolylpurine as an Artificial Nucleobase for
Metal-Mediated Base Pairing in DNA Duplexes
J. Christian Léon1, Indranil Sinha1,2,†and Jens Müller1,2,*1 Institut für Anorganische und Analytische Chemie, Westfälische Wilhelms-Universität Münster, Corrensstraße 30, 48149 Münster, Germany; j_leon02@uni-muenster.de (J.C.L.);
indranilsinha14@gmail.com (I.S.)
2 NRW Graduate School of Chemistry, Westfälische Wilhelms-Universität Münster, Corrensstraße 30, 48149 Münster, Germany
* Correspondence: mueller.j@uni-muenster.de; Tel.: +49-251-83-36006
† Current address: Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), 291 Daehak-ro, Yuseong-gu, 305-701 Daejeon, Korea.
Academic Editor: Eva Freisinger
Received: 9 March 2016; Accepted: 5 April 2016; Published: 14 April 2016
Abstract:The artificial nucleobase 6-pyrazol-1-yl-purine (6PP) has been investigated with respect to its usability in metal-mediated base pairing. As was shown by temperature-dependent UV spectroscopy, 6PP may form weakly stabilizing 6PP–Ag(I)–6PP homo base pairs. Interestingly, 6PP can be used to selectively recognize a complementary pyrimidine nucleobase. The addition of Ag(I) to a DNA duplex comprising a central 6PP:C mispair (C = cytosine) leads to a slight destabilization of the duplex. In contrast, a stabilizing 6PP–Ag(I)–T base pair is formed with a complementary thymine (T) residue. It is interesting to note that 6PP is capable of differentiating between the pyrimidine moieties despite the fact that it is not as sterically crowded as 6-(3,5-dimethylpyrazol-1-yl)purine, an artificial nucleobase that had previously been suggested for the recognition of nucleic acid sequences via the formation of a metal-mediated base pair. Hence, the additional methyl groups of 6-(3,5-dimethylpyrazol-1-yl)purine may not be required for the specific recognition of the complementary nucleobase.
Keywords:cytosine; metal-mediated base pair; purine; thymine
1. Introduction
Nucleic acids are increasingly being used as building blocks in nanotechnology, owing to their superb and highly predictable self-assembly, their stiffness, and the ease of their modification [1]. An important way to introduce metal-based functionality into nucleic acids is the use of metal-mediated base pairs. In these artificial base pairs, coordinative bonds to one or two central metal ions formally replace the hydrogen bonds usually found between complementary nucleobases [2–5]. As confirmed by several structural studies, the metal ions align along the helical axis inside B-DNA duplexes [6–8]. Similarly, Z-DNA and A-RNA duplexes are also compatible with the formation of metal-mediated base pairs [9–11]. The resulting metal-containing nucleic acids have found manifold applications, including their use as sensors [12], in expanding the genetic code [13], in modifying the charge transfer capabilities of DNA [14,15], in the generation of metal nanoclusters [16], and in the recognition of other nucleic acid sequences [17].
In the latter context, a series of purine derivatives with appended donor moieties have recently been proposed as artificial nucleobases for the specific recognition of a complementary canonical nucleobase via the formation of a metal-mediated base pair [18–21]. For one of these derivatives, i.e., 6-(3,5-dimethylpyrazol-1-yl)purine, single-crystal X-ray diffraction analysis of a model nucleobase
confirmed the suggested binding pattern of the transition metal ions, namely, via the pyrazole nitrogen atom and the purine N7 position [22]. The same study also indicated that this binding pattern is retained when formally removing the methyl groups from the pyrazole rings, i.e., when using 6-pyrazol-1-yl-purine. Moreover, due to the lack of a steric clash between the (absent) methyl groups, the model nucleobase 9-methyl-6-pyrazol-1-yl-purine was shown to be able to form homoleptic 2:1 complexes with Cu(II) and Ag(I) [22]. As this artificial purine derivative had never been tested as a nucleobase for metal-mediated base pairing inside a DNA duplex, we set out to investigate its corresponding properties. This study reports that 6-pyrazolyl-1-yl-purine (6PP) is able to form an Ag(I)-mediated base pair with a complementary thymine residue, but that stabilizing homo base pairs of the type 6PP–Mn+–6PP are not formed inside a DNA duplex.
2. Results
2.1. Synthesis of the Deoxyribonucleoside and the DNA Duplex
The artificial nucleobase 6-pyrazol-1-yl-purine 1 was synthesized by stirring 6-chloropurine in neat pyrazole at 150 ˝C (Scheme 1) [23]. Reaction of 1 with Hoffer’s chloro sugar gave the
β isomer of the p-toluoyl-protected deoxyribonucleoside 2. Finally, deprotection by means of aqueous methanolic ammonia resulted in the formation of the desired 6PP deoxyribonucleoside 3. To enable its use in automated DNA oligonucleotide solid-phase synthesis, the hydroxyl groups were protected in two subsequent steps with 4,41-dimethoxytrityl (51-OH) and
2-cyanoethyl-N,N-diisopropyl-phosphoramidite (31-OH) to give the 6PP phosphoramidite building block 5.
Int. J. Mol. Sci. 2016, 17, 554 2 of 10
nitrogen atom and the purine N7 position [22]. The same study also indicated that this binding pattern is retained when formally removing the methyl groups from the pyrazole rings, i.e., when using 6-pyrazol-1-yl-purine. Moreover, due to the lack of a steric clash between the (absent) methyl groups, the model nucleobase 9-methyl-6-pyrazol-1-yl-purine was shown to be able to form homoleptic 2:1 complexes with Cu(II) and Ag(I) [22]. As this artificial purine derivative had never been tested as a nucleobase for metal-mediated base pairing inside a DNA duplex, we set out to investigate its corresponding properties. This study reports that 6-pyrazolyl-1-yl-purine (6PP) is able to form an Ag(I)-mediated base pair with a complementary thymine residue, but that stabilizing homo base pairs of the type 6PP–Mn+–6PP are not formed inside a DNA duplex.
2. Results
2.1. Synthesis of the Deoxyribonucleoside and the DNA Duplex
The artificial nucleobase 6-pyrazol-1-yl-purine 1 was synthesized by stirring 6-chloropurine in neat pyrazole at 150 °C (Scheme 1) [23]. Reaction of 1 with Hoffer’s chloro sugar gave the β isomer of the p-toluoyl-protected deoxyribonucleoside 2. Finally, deprotection by means of aqueous methanolic ammonia resulted in the formation of the desired 6PP deoxyribonucleoside 3. To enable its use in automated DNA oligonucleotide solid-phase synthesis, the hydroxyl groups were protected in two subsequent steps with 4,4′-dimethoxytrityl (5′-OH) and 2-cyanoethyl-N,N-diisopropyl-phosphoramidite (3′-OH) to give the 6PP 2-cyanoethyl-N,N-diisopropyl-phosphoramidite building block 5.
Scheme 1. Synthesis of the 6-pyrazolyl-1-yl-purine (6PP) deoxyribonucleoside (3) via free 6PP (1) and
p-toluoyl-protected 6PP deoxyribonucleoside (2). a) Neat, 150 °C, 1 h; b) first step: NaH (1.6 equiv.),
CH3CN, 0 °C, 1 h, second step: Hoffer’s chloro sugar, toluene, overnight; c) aqueous NH3, methanol,
RT, 4 h.
The oligonucleotide duplex investigated in this study is shown in Scheme 2. It was chosen because several other metal-mediated base pairs have already been characterized in this sequence context, thereby enabling a direct comparison with other systems. The duplex comprises 12 natural base pairs plus one central artificial base pair (denoted as X:Y in Scheme 2).
Scheme 2. Oligonucleotide duplex under investigation.
2.2. Spectroscopic Characterization of the Metal-Containing DNA Duplex
2.2.1. Homo Base Pair (6PP:6PP)
As the structural studies using the model nucleobase 9-methyl-6-pyrazol-1-yl-purine had suggested the possibility of the formation of a metal-mediated homo base pair from 6PP [22], we initially investigated the incorporation of metal ions into a DNA duplex comprising a central 6PP:6PP mispair. The formation of the anticipated metal-mediated base pair was examined by determining the melting temperatures of the duplex Tm in the presence of increasing amounts of suitable metal
Scheme 1.Synthesis of the 6-pyrazolyl-1-yl-purine (6PP) deoxyribonucleoside (3) via free 6PP (1) and p-toluoyl-protected 6PP deoxyribonucleoside (2). a) Neat, 150˝C, 1 h; b) first step: NaH (1.6 equiv.), CH3CN, 0˝C, 1 h, second step: Hoffer’s chloro sugar, toluene, overnight; c) aqueous NH3, methanol, RT, 4 h.
The oligonucleotide duplex investigated in this study is shown in Scheme2. It was chosen because several other metal-mediated base pairs have already been characterized in this sequence context, thereby enabling a direct comparison with other systems. The duplex comprises 12 natural base pairs plus one central artificial base pair (denoted as X:Y in Scheme2).
nitrogen atom and the purine N7 position [22]. The same study also indicated that this binding pattern is retained when formally removing the methyl groups from the pyrazole rings, i.e., when using 6-pyrazol-1-yl-purine. Moreover, due to the lack of a steric clash between the (absent) methyl groups, the model nucleobase 9-methyl-6-pyrazol-1-yl-purine was shown to be able to form homoleptic 2:1 complexes with Cu(II) and Ag(I) [22]. As this artificial purine derivative had never been tested as a nucleobase for metal-mediated base pairing inside a DNA duplex, we set out to investigate its corresponding properties. This study reports that 6-pyrazolyl-1-yl-purine (6PP) is able to form an Ag(I)-mediated base pair with a complementary thymine residue, but that stabilizing homo base pairs of the type 6PP–Mn+–6PP are not formed inside a DNA duplex.
2. Results
2.1. Synthesis of the Deoxyribonucleoside and the DNA Duplex
The artificial nucleobase 6-pyrazol-1-yl-purine 1 was synthesized by stirring 6-chloropurine in neat pyrazole at 150 °C (Scheme 1) [23]. Reaction of 1 with Hoffer’s chloro sugar gave the β isomer of the p-toluoyl-protected deoxyribonucleoside 2. Finally, deprotection by means of aqueous methanolic ammonia resulted in the formation of the desired 6PP deoxyribonucleoside 3. To enable its use in automated DNA oligonucleotide solid-phase synthesis, the hydroxyl groups were protected in two subsequent steps with 4,4′-dimethoxytrityl (5′-OH) and 2-cyanoethyl-N,N-diisopropyl-phosphoramidite (3′-OH) to give the 6PP 2-cyanoethyl-N,N-diisopropyl-phosphoramidite building block 5.
Scheme 1. Synthesis of the 6-pyrazolyl-1-yl-purine (6PP) deoxyribonucleoside (3) via free 6PP (1) and
p-toluoyl-protected 6PP deoxyribonucleoside (2). a) Neat, 150 °C, 1 h; b) first step: NaH (1.6 equiv.),
CH3CN, 0 °C, 1 h, second step: Hoffer’s chloro sugar, toluene, overnight; c) aqueous NH3, methanol,
RT, 4 h.
The oligonucleotide duplex investigated in this study is shown in Scheme 2. It was chosen because several other metal-mediated base pairs have already been characterized in this sequence context, thereby enabling a direct comparison with other systems. The duplex comprises 12 natural base pairs plus one central artificial base pair (denoted as X:Y in Scheme 2).
Scheme 2. Oligonucleotide duplex under investigation.
2.2. Spectroscopic Characterization of the Metal-Containing DNA Duplex
2.2.1. Homo Base Pair (6PP:6PP)
As the structural studies using the model nucleobase 9-methyl-6-pyrazol-1-yl-purine had suggested the possibility of the formation of a metal-mediated homo base pair from 6PP [22], we initially investigated the incorporation of metal ions into a DNA duplex comprising a central 6PP:6PP mispair. The formation of the anticipated metal-mediated base pair was examined by determining the melting temperatures of the duplex Tm in the presence of increasing amounts of suitable metal
Scheme 2.Oligonucleotide duplex under investigation.
2.2. Spectroscopic Characterization of the Metal-Containing DNA Duplex 2.2.1. Homo Base Pair (6PP:6PP)
As the structural studies using the model nucleobase 9-methyl-6-pyrazol-1-yl-purine had suggested the possibility of the formation of a metal-mediated homo base pair from 6PP [22], we initially investigated the incorporation of metal ions into a DNA duplex comprising a central
6PP:6PP mispair. The formation of the anticipated metal-mediated base pair was examined by determining the melting temperatures of the duplex Tmin the presence of increasing amounts of
suitable metal ions. As the formation of coordinate bonds between the artificial nucleobases and the metal ion typically leads to an increased stability of the duplex [2], monitoring the Tm often
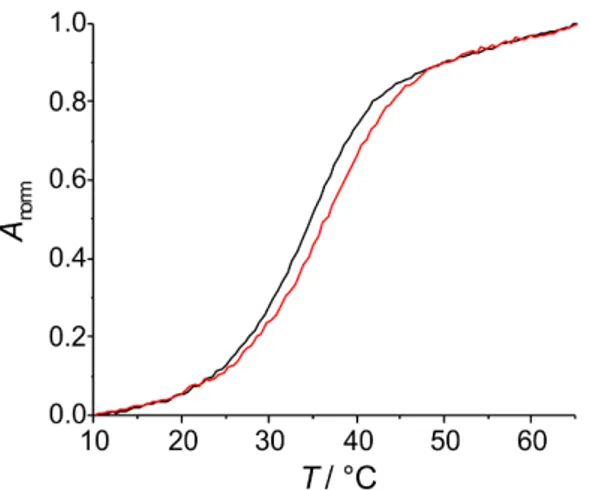
represents a good indication for the formation of a metal-mediated base pair. Exceptions from this rule are rare, but are sometimes found in the case of non-planar metal-mediated base pairs [24,25] or when the metal-free duplex is exceptionally stable [26]. Figure1shows the melting curves of the duplex comprising a 6PP:6PP base pair in the absence and presence of one equivalent of AgNO3.
Prior to the addition of AgNO3, the duplex melts at 34.8˝C. This melting temperature is in the
upper range of melting temperatures reported for the same sequence with other artificial nucleobases (21.7–39.1˝C) [27] but is significantly below that of a duplex comprising a central A:T or G:C base pair
(42.5, 45.4˝C) [28]. In the presence of one Ag(I) per duplex, T
mincreases by 2.5˝C to a final value of
37.3˝C. This rather small increase does not unambiguously confirm the formation of a metal-mediated
base pair. However, considering that previous studies showed that a small thermal stabilization does not rule out the formation of a metal-mediated base pair [24,25], in particular when the base pair is non-planar, no final conclusion regarding the formation of a 6PP–Ag(I)–6PP base pair can be drawn.
Int. J. Mol. Sci. 2016, 17, 554 3 of 10
ions. As the formation of coordinate bonds between the artificial nucleobases and the metal ion typically leads to an increased stability of the duplex [2], monitoring the Tm often represents a good
indication for the formation of a metal-mediated base pair. Exceptions from this rule are rare, but are sometimes found in the case of non-planar metal-mediated base pairs [24,25] or when the metal-free duplex is exceptionally stable [26]. Figure 1 shows the melting curves of the duplex comprising a 6PP:6PP base pair in the absence and presence of one equivalent of AgNO3. Prior to the addition
of AgNO3, the duplex melts at 34.8 °C. This melting temperature is in the upper range of melting
temperatures reported for the same sequence with other artificial nucleobases (21.7–39.1 °C) [27] but is significantly below that of a duplex comprising a central A:T or G:C base pair (42.5, 45.4 °C) [28]. In the presence of one Ag(I) per duplex, Tm increases by 2.5 °C to a final value of 37.3 °C. This rather
small increase does not unambiguously confirm the formation of a metal-mediated base pair. However, considering that previous studies showed that a small thermal stabilization does not rule out the formation of a metal-mediated base pair [24,25], in particular when the base pair is non-planar, no final conclusion regarding the formation of a 6PP–Ag(I)–6PP base pair can be drawn.
Figure 1. UV melting curves of the duplex comprising a central 6PP:6PP base pair in the absence (black) and presence (red) of one equivalent of AgNO3.
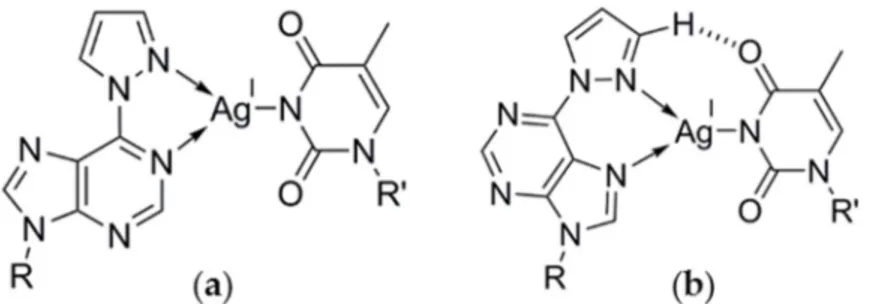
A putative 6PP–Ag(I)–6PP base pair could exist with coordinate bonds involving the purine N1 atom, i.e., via the Watson–Crick edge (Scheme 3a), or the purine N7 atom, i.e., via the Hoogsteen edge (Scheme 3b). Both types of metal-mediated base pairing patterns have previously been reported for other purine derivatives within antiparallel-stranded duplexes [29–31]. Considering that all metal complexes of the model nucleobase showed coordination via N7 [22], this binding pattern (Scheme 3b) is also the more likely one for the putative 6PP–Ag(I)–6PP base pair.
Scheme 3. Possible structures of the putative 6PP–Ag(I)–6PP base pair (R, R′ = DNA backbone). (a) Watson–Crick-type binding via N1; (b) Hoogsteen-type binding via N7.
Attempts to introduce other metal ions led to an even smaller change in the case of Cu(II) (∆Tm = +1.0 °C) and no change at all for Ni(II), Hg(II), and Zn(II). For the latter three metal ions, the
formation of a metal-mediated base pair can be excluded. For Ni(II), this is surprising, because the closely related 6-pyridylpurine had been reported to form exceptionally stabilizing Ni(II)-mediated homo base pairs (∆Tm = 18 °C) [30].
Figure 1.UV melting curves of the duplex comprising a central 6PP:6PP base pair in the absence (black) and presence (red) of one equivalent of AgNO3.
A putative 6PP–Ag(I)–6PP base pair could exist with coordinate bonds involving the purine N1 atom, i.e., via the Watson–Crick edge (Scheme3a), or the purine N7 atom, i.e., via the Hoogsteen edge (Scheme3b). Both types of metal-mediated base pairing patterns have previously been reported for other purine derivatives within antiparallel-stranded duplexes [29–31]. Considering that all metal complexes of the model nucleobase showed coordination via N7 [22], this binding pattern (Scheme3b) is also the more likely one for the putative 6PP–Ag(I)–6PP base pair.
Int. J. Mol. Sci. 2016, 17, 554 3 of 10
ions. As the formation of coordinate bonds between the artificial nucleobases and the metal ion typically leads to an increased stability of the duplex [2], monitoring the Tm often represents a good
indication for the formation of a metal-mediated base pair. Exceptions from this rule are rare, but are sometimes found in the case of non-planar metal-mediated base pairs [24,25] or when the metal-free duplex is exceptionally stable [26]. Figure 1 shows the melting curves of the duplex comprising a 6PP:6PP base pair in the absence and presence of one equivalent of AgNO3. Prior to the addition
of AgNO3, the duplex melts at 34.8 °C. This melting temperature is in the upper range of melting
temperatures reported for the same sequence with other artificial nucleobases (21.7–39.1 °C) [27] but is significantly below that of a duplex comprising a central A:T or G:C base pair (42.5, 45.4 °C) [28]. In the presence of one Ag(I) per duplex, Tm increases by 2.5 °C to a final value of 37.3 °C. This rather
small increase does not unambiguously confirm the formation of a metal-mediated base pair. However, considering that previous studies showed that a small thermal stabilization does not rule out the formation of a metal-mediated base pair [24,25], in particular when the base pair is non-planar, no final conclusion regarding the formation of a 6PP–Ag(I)–6PP base pair can be drawn.
Figure 1. UV melting curves of the duplex comprising a central 6PP:6PP base pair in the absence (black) and presence (red) of one equivalent of AgNO3.
A putative 6PP–Ag(I)–6PP base pair could exist with coordinate bonds involving the purine N1 atom, i.e., via the Watson–Crick edge (Scheme 3a), or the purine N7 atom, i.e., via the Hoogsteen edge (Scheme 3b). Both types of metal-mediated base pairing patterns have previously been reported for other purine derivatives within antiparallel-stranded duplexes [29–31]. Considering that all metal complexes of the model nucleobase showed coordination via N7 [22], this binding pattern (Scheme 3b) is also the more likely one for the putative 6PP–Ag(I)–6PP base pair.
Scheme 3. Possible structures of the putative 6PP–Ag(I)–6PP base pair (R, R′ = DNA backbone). (a) Watson–Crick-type binding via N1; (b) Hoogsteen-type binding via N7.
Attempts to introduce other metal ions led to an even smaller change in the case of Cu(II) (∆Tm = +1.0 °C) and no change at all for Ni(II), Hg(II), and Zn(II). For the latter three metal ions, the
formation of a metal-mediated base pair can be excluded. For Ni(II), this is surprising, because the closely related 6-pyridylpurine had been reported to form exceptionally stabilizing Ni(II)-mediated homo base pairs (∆Tm = 18 °C) [30].
Scheme 3. Possible structures of the putative 6PP–Ag(I)–6PP base pair (R, R1 = DNA backbone). (a) Watson–Crick-type binding via N1; (b) Hoogsteen-type binding via N7.
Attempts to introduce other metal ions led to an even smaller change in the case of Cu(II) (∆Tm= +1.0˝C) and no change at all for Ni(II), Hg(II), and Zn(II). For the latter three metal ions,
the formation of a metal-mediated base pair can be excluded. For Ni(II), this is surprising, because the closely related 6-pyridylpurine had been reported to form exceptionally stabilizing Ni(II)-mediated homo base pairs (∆Tm= 18˝C) [30].
2.2.2. Hetero Base Pairs (6PP:C and 6PP:T)
Following the initial notion that 6-(3,5-dimethylpyrazol-1-yl)-purine may be able to specifically recognize a canonical nucleobase [18], we also investigated this possibility with the sterically less demanding 6-pyrazol-1-yl-purine 6PP. As 6PP is a purine derivative, we limited our investigation to the base pairing with the pyrimidine nucleobases cytosine and thymine. Moreover, as only Ag(I) had a significant influence on the melting temperature of the duplex comprising 6PP:6PP, we focused on the use of AgNO3in the study of the possible metal-mediated 6PP:C and 6PP:T base pairs. Figure2shows
the melting curves of the duplexes containing these artificial base pairs in their center. As thymine is protonated at N3, the study involving the thymine-containing base pair was performed at an elevated pH in order to facilitate deprotonation and subsequent coordination to Ag(I).
Int. J. Mol. Sci. 2016, 17, 554 4 of 10
2.2.2. Hetero Base Pairs (6PP:C and 6PP:T)
Following the initial notion that 6-(3,5-dimethylpyrazol-1-yl)-purine may be able to specifically recognize a canonical nucleobase [18], we also investigated this possibility with the sterically less demanding 6-pyrazol-1-yl-purine 6PP. As 6PP is a purine derivative, we limited our investigation to the base pairing with the pyrimidine nucleobases cytosine and thymine. Moreover, as only Ag(I) had a significant influence on the melting temperature of the duplex comprising 6PP:6PP, we focused on the use of AgNO3 in the study of the possible metal-mediated 6PP:C and 6PP:T base
pairs. Figure 2 shows the melting curves of the duplexes containing these artificial base pairs in their center. As thymine is protonated at N3, the study involving the thymine-containing base pair was performed at an elevated pH in order to facilitate deprotonation and subsequent coordination to Ag(I).
Figure 2. UV melting curves of the duplex comprising a central 6PP:pyrimidine base pair in the presence of increasing amounts of AgNO3 (black: 0 equiv.; red: 1. equiv.; blue: 2 equiv.; orange: 3
equiv.) (a) 6PP:C (pH 6.8); (b) 6PP:T (pH 9.0). The arrow indicates the direction of the changes upon the addition of AgNO3. The inset shows the increase in Tm depending on the amount of added AgNO3.
In the absence of Ag(I), both duplexes melt at around 29 °C. As expected, the duplex is slightly destabilized at pH 9.0 with respect to the one at pH 6.8 (28.1 °C vs. 30.1 °C). The average melting temperature is significantly lower than that of the duplex with a central 6PP:6PP base pair and reflects the importance of π stacking interactions in stabilizing a nucleic acid duplex. Apparently, the reduced π surface of the pyrimidine nucleobases compared with the purine derivative effect this decrease in stability.
It is interesting to note that the addition of AgNO3 to the duplex containing a central 6PP:C base
pair (Figure 2a) slightly destabilizes the duplex even further (∆Tm = −2 °C). In contrast, when Ag(I) is
added to the duplex with a central 6PP:T base pair, a significant increase in Tm is observed
(∆Tm = 4.3 °C). Moreover, the addition of excess AgNO3 does not significantly influence the melting
temperature anymore. This is a clear indication of the formation of a mononuclear Ag(I)-mediated base pair, as the extra stability is conferred by the incorporation of the first Ag(I) only [2]. Similarly, a plot of the circular dichroism (CD) spectra of the duplex comprising the central 6PP:T base pair in the presence of increasing amounts of AgNO3 shows negligible changes during the stepwise addition
of the first equivalent but significant changes in the presence of excess Ag(I) (Figure 3). This indicates that the overall duplex structure is not disrupted by the formation of the 6PP–Ag(I)–T base pair and that excess Ag(I) also binds to duplex, albeit at other positions [28,32].
Figure 2. UV melting curves of the duplex comprising a central 6PP:pyrimidine base pair in the presence of increasing amounts of AgNO3 (black: 0 equiv.; red: 1. equiv.; blue: 2 equiv.; orange: 3 equiv.) (a) 6PP:C (pH 6.8); (b) 6PP:T (pH 9.0). The arrow indicates the direction of the changes upon the addition of AgNO3. The inset shows the increase in Tmdepending on the amount of added AgNO3. In the absence of Ag(I), both duplexes melt at around 29˝C. As expected, the duplex is slightly
destabilized at pH 9.0 with respect to the one at pH 6.8 (28.1˝C vs. 30.1˝C). The average melting
temperature is significantly lower than that of the duplex with a central 6PP:6PP base pair and reflects the importance of π stacking interactions in stabilizing a nucleic acid duplex. Apparently, the reduced πsurface of the pyrimidine nucleobases compared with the purine derivative effect this decrease in stability.
It is interesting to note that the addition of AgNO3to the duplex containing a central 6PP:C
base pair (Figure2a) slightly destabilizes the duplex even further (∆Tm= ´2˝C). In contrast, when
Ag(I) is added to the duplex with a central 6PP:T base pair, a significant increase in Tmis observed
(∆Tm= 4.3˝C). Moreover, the addition of excess AgNO3does not significantly influence the melting
temperature anymore. This is a clear indication of the formation of a mononuclear Ag(I)-mediated base pair, as the extra stability is conferred by the incorporation of the first Ag(I) only [2]. Similarly, a plot of the circular dichroism (CD) spectra of the duplex comprising the central 6PP:T base pair in the presence of increasing amounts of AgNO3shows negligible changes during the stepwise addition
that the overall duplex structure is not disrupted by the formation of the 6PP–Ag(I)–T base pair and that excess Ag(I) also binds to duplex, albeit at other positions [Int. J. Mol. Sci. 2016, 17, 554 28,32]. 5 of 10
Figure 3. Circular dichroism (CD) spectra of the duplex comprising a central 6PP:T base pair in the presence of increasing amounts of AgNO3 (0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3 equiv.). The arrow indicates the
direction of the changes upon the addition of AgNO3.
Scheme 4 shows possible structures of the 6PP–Ag(I)–T base pair. Similarly to 6PP–Ag(I)–6PP, the Ag(I) ion may bind via N1 or via N7. In both cases, a [2+1] coordination environment is proposed for the metal ion, in analogy to what has been found in other Ag(I)-mediated base pairs [26,33]. Weak additional binding via one of the carbonyl oxygen atoms cannot be ruled out though. As the crystal structures of the complexes of the corresponding model nucleobase had shown that N7 is the preferred metal binding site of 6PP, the arrangement shown in Scheme 4b appears to be more likely. In this structure, the possible formation of a C–H···O hydrogen bond involving an aromatic hydrogen atom of the pyrazole moiety may even reinforce the preference of the metal ion for N7.
Scheme 4. Possible structures of the 6PP–Ag(I)–T base pair (R, R′ = DNA backbone). (a) Watson–Crick-type binding via N1; (b) Hoogsteen-type binding via N7.
3. Discussion
The data presented here show that 6PP represents a valuable addition to the list of artificial nucleobases for metal-mediated base pairing. Out of the several substituted purine derivatives published to date [18,19,28,30,31,34–37], it adds to those ones with an N,N-donor set [18,30]. Its putative metal-mediated homo base pairs do not evoke a strong thermal stabilization of the DNA duplex. In contrast, it readily forms a stabilizing Ag(I)-mediated base pair with the canonical nucleobase thymine. Even though it is not as sterically crowded as its derivative 6-(3,5-dimethylpyrazol-1-yl)purine, 6PP has the potential to discriminate between a complementary cytosine and a thymine via the formation of an Ag(I)-mediated base pair. A similar, albeit not as pronounced, stability trend has been reported for the metal-mediated base pairs formed from 6-(3,5-dimethylpyrazol-1-yl)purine and the pyrimidine nucleobases in 2′-O-methyl RNA oligonucleotides in the presence of Cu(II) [18]. The similarities between 6-(3,5-dimethylpyrazol-1-yl)purine and 6-pyrazol-1-yl-purine suggest that the methyl substituents on the pyrazole moiety may not be required to achieve the specific recognition of a complementary nucleobase.
Figure 3.Circular dichroism (CD) spectra of the duplex comprising a central 6PP:T base pair in the presence of increasing amounts of AgNO3(0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3 equiv.). The arrow indicates the direction of the changes upon the addition of AgNO3.
Scheme4shows possible structures of the 6PP–Ag(I)–T base pair. Similarly to 6PP–Ag(I)–6PP, the Ag(I) ion may bind via N1 or via N7. In both cases, a [2+1] coordination environment is proposed for the metal ion, in analogy to what has been found in other Ag(I)-mediated base pairs [26,33]. Weak additional binding via one of the carbonyl oxygen atoms cannot be ruled out though. As the crystal structures of the complexes of the corresponding model nucleobase had shown that N7 is the preferred metal binding site of 6PP, the arrangement shown in Scheme4b appears to be more likely. In this structure, the possible formation of a C–H¨ ¨ ¨ O hydrogen bond involving an aromatic hydrogen atom of the pyrazole moiety may even reinforce the preference of the metal ion for N7.
Int. J. Mol. Sci. 2016, 17, 554 5 of 10
Figure 3. Circular dichroism (CD) spectra of the duplex comprising a central 6PP:T base pair in the presence of increasing amounts of AgNO3 (0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3 equiv.). The arrow indicates the
direction of the changes upon the addition of AgNO3.
Scheme 4 shows possible structures of the 6PP–Ag(I)–T base pair. Similarly to 6PP–Ag(I)–6PP, the Ag(I) ion may bind via N1 or via N7. In both cases, a [2+1] coordination environment is proposed for the metal ion, in analogy to what has been found in other Ag(I)-mediated base pairs [26,33]. Weak additional binding via one of the carbonyl oxygen atoms cannot be ruled out though. As the crystal structures of the complexes of the corresponding model nucleobase had shown that N7 is the preferred metal binding site of 6PP, the arrangement shown in Scheme 4b appears to be more likely. In this structure, the possible formation of a C–H···O hydrogen bond involving an aromatic hydrogen atom of the pyrazole moiety may even reinforce the preference of the metal ion for N7.
Scheme 4. Possible structures of the 6PP–Ag(I)–T base pair (R, R′ = DNA backbone). (a) Watson–Crick-type binding via N1; (b) Hoogsteen-type binding via N7.
3. Discussion
The data presented here show that 6PP represents a valuable addition to the list of artificial nucleobases for metal-mediated base pairing. Out of the several substituted purine derivatives published to date [18,19,28,30,31,34–37], it adds to those ones with an N,N-donor set [18,30]. Its putative metal-mediated homo base pairs do not evoke a strong thermal stabilization of the DNA duplex. In contrast, it readily forms a stabilizing Ag(I)-mediated base pair with the canonical nucleobase thymine. Even though it is not as sterically crowded as its derivative 6-(3,5-dimethylpyrazol-1-yl)purine, 6PP has the potential to discriminate between a complementary cytosine and a thymine via the formation of an Ag(I)-mediated base pair. A similar, albeit not as pronounced, stability trend has been reported for the metal-mediated base pairs formed from 6-(3,5-dimethylpyrazol-1-yl)purine and the pyrimidine nucleobases in 2′-O-methyl RNA oligonucleotides in the presence of Cu(II) [18]. The similarities between 6-(3,5-dimethylpyrazol-1-yl)purine and 6-pyrazol-1-yl-purine suggest that the methyl substituents on the pyrazole moiety may not be required to achieve the specific recognition of a complementary nucleobase.
Scheme 4.Possible structures of the 6PP–Ag(I)–T base pair (R, R1= DNA backbone). (a) Watson–Crick-type binding via N1; (b) Hoogsteen-Watson–Crick-type binding via N7.
3. Discussion
The data presented here show that 6PP represents a valuable addition to the list of artificial nucleobases for metal-mediated base pairing. Out of the several substituted purine derivatives published to date [18,19,28,30,31,34–37], it adds to those ones with an N,N-donor set [18,30]. Its putative metal-mediated homo base pairs do not evoke a strong thermal stabilization of the DNA duplex. In contrast, it readily forms a stabilizing Ag(I)-mediated base pair with the canonical nucleobase thymine. Even though it is not as sterically crowded as its derivative 6-(3,5-dimethylpyrazol-1-yl) purine, 6PP has the potential to discriminate between a complementary cytosine and a thymine via the formation of an Ag(I)-mediated base pair. A similar, albeit not as pronounced, stability trend has been reported for the metal-mediated base pairs formed from 6-(3,5-dimethylpyrazol-1-yl)purine and the pyrimidine nucleobases in 21-O-methyl RNA oligonucleotides in the presence of Cu(II) [18].
The similarities between 6-(3,5-dimethylpyrazol-1-yl)purine and 6-pyrazol-1-yl-purine suggest that the methyl substituents on the pyrazole moiety may not be required to achieve the specific recognition of a complementary nucleobase.
4. Materials and Methods
4.1. Oligonucleotide Synthesis and Characterization
Phosphoramidites required for the synthesis of the oligonucleotide sequences were from Glen Research. Syntheses of the oligonucleotide strands were performed on a K & A Laborgeräte H8 DNA/RNA synthesizer in the DMT-off mode by following standard protocols. Post-synthesis, the oligonucleotides were cleaved from the solid support and deprotected by treating them with tert-butylamine/MeOH/NH3 (1/2/1) for 4 h at 60 ˝C. Thereafter, they were purified by
denaturing urea polyacrylamide gel electrophoresis (gel solution: 7 M urea, 1 M TBE buffer, 18% polyacrylamide:bisacrylamide (29:1); loading buffer: 11.8 M urea, 42 mM Tris/HCl (pH 7.5), 0.83 mM EDTA (pH 8.0), 8% sucrose, 0.08% dye (xylene cyanol, bromophenol blue)). After purification, the oligonucleotides were desalted with NAP10 columns. The desalted oligonucleotides were characterized by MALDI-TOF mass spectrometry (51-d(GAG GGA XAG AAA G)-31: calcd. for [M + H]+:
4157 Da, found: 4156 Da; 51-d(CTC CCT XTC TTT C)-31: calcd. for [M + H]+: 3863 Da, found: 3862 Da;
51-d(CTC CCT TTC TTT C)-31: calcd. for [M + H]+: 3803 Da, found: 3802 Da; 51-d(CTC CCT CTC
TTT C)-31: calcd. for [M + H]+: 3789 Da, found: 3787 Da). MALDI-TOF mass spectra were recorded
on a Bruker Reflex IV instrument using a 3-hydroxypicolinic acid/ammonium citrate matrix. High resolution (positive mode) mass spectra were obtained on a Waters Q-Tof Premier Micromass HAB 213 mass spectrometer or on an LTQ Orbitrap XL (Thermo Scientific, Bremen, Germany), equipped with the static nanospray probe (slightly modified to use self-drawn glass nanospray capillaries, spray voltage 1.0–1.4 kV, capillary temperature 200˝C, tube lens approx. 50–120 V). During the
quantification of the oligonucleotides, a molar extinction coefficient ε260of 4.8 cm2¨ µmol´1was used
for 6PP deoxyribonucleoside. 4.2. Spectroscopy
NMR spectra were recorded using Bruker Avance(I) 400 and Bruker Avance(III) 400 spectrometers. Chemical shifts were recorded with reference to residual solvent peak in DMSO-d6 (1H NMR
δ= 2.49 ppm,13C NMR δ = 39.5 ppm), CDCl3 (1H NMR δ = 7.26 ppm, 13C NMR δ = 77.2 ppm),
CD3CN (1H NMR δ = 1.94 ppm,13C NMR δ = 118.3 ppm), or with respect to trimethylsilyl propane
sulfonate (D2O, δ = 0 ppm). UV melting experiments were carried out on a UV spectrometer CARY
100 Bio using a 1-cm quartz cuvette. The UV melting profiles were measured at 260 nm in buffer at pH 6.8 (1 µM oligonucleotide duplex, 150 mM NaClO4, 5 mM MOPS) or pH 9.0 (1 µM oligonucleotide
duplex, 150 mM NaClO4, 5 mM borate), either in absence or in presence of AgNO3, at a heating rate of
1˝C¨ min–1with data being recorded at an interval of 0.5˝C. Prior to each measurement, the sample
was equilibrated by heating it to 60˝C followed by cooling to 10˝C at a rate of 1˝C¨ min–1. Melting
temperatures were determined from the maxima of the first derivatives of the melting curves. 4.3. 6-(1H-Pyrazol-1-yl)-9H-purine 1
Compound 1 was synthesized according to a literature procedure [23]. Its1H NMR spectrum agreed well with the literature data. Additional characterization data are as follows. 13C NMR
(101 MHz, DMSO-d6), δ: 151.1 (C2p), 146.8 (C4p), 144.2 (C8p), 138.0 (C6p), 130.9 (C5p), 127.7 (C3*),
115.4 (C5*), 109.2 (C4*) ppm. ESI-MS m/z: 187.0727 [M + H]+(calcd. 187.0732), [M + Na]+209.0546 (calcd. 209.0552). Elemental analysis (%): found: C 51.6, H 3.3, N 44.7 calcd. for C8H6N6: C 51.6, H 3.3,
4.4. 9-(2-Deoxy-3,5-di-O-p-toluoyl-β-D-erythro-pentofuranosyl)-6-(1H-pyrazol-1-yl)-9H-purine 2
6-(1H-Pyrazol-1-yl)-9H-purine 1 (0.500 g, 2.69 mmol) was suspended in dry acetonitrile (20 mL), and NaH (0.170 g of a 60% suspension on oil, 4.26 mmol) was added. The resulting mixture was stirred for 1 h under ice cooling, followed by the addition of Hoffer’s chloro sugar [38] (1.25 g, 3.22 mmol), which was previously suspended in dry toluene (20 mL), over a period of 20 min in four portions. The solution was stirred overnight and the solvent evaporated. The oily residue was purified by silica gel column chromatography (cyclohexane (8):CH2Cl2(1):EtOAc (4):Et3N (3)) to produce an off-white
foam of the desired β anomer 2. Formation of the undesired α anomer was not observed. Yield: 0.673 g (48%).1H NMR (400 MHz, CDCl3), δ: 9.00 (d, 1H, H5*), 8.75 (s, 1H, H2p), 8.49 (s, 1H, H8p), 7.97 (d, 1H,
H3*), 7.93 (d, 2H, o-H (Tol)), 7.58 (d, 2H, o-H (Tol)), 7.25 (d, 2H, m-H (Tol)), 7.10 (d, 2H, m-H (Tol)), 6.71 (dd, 1H, H11), 6.56 (s, 1H, H4*), 5.70 (m, 1H, H31), 4.92 (m, 1H, H41), 4.62 (m, 2H, H51/H511), 3.14 (m, 1H,
H21or H211), 3.09 (m, 1H, H21or H211), 2.40 (s, 3H, CH
3), 2.31 (s, 3H, CH3) ppm.13C NMR (101 MHz,
CDCl3), δ: 166.0 (C=O), 165.7 (C=O), 153.2 (C4p), 152.0 (C2p), 147.2 (C6p), 144.5 (Tol), 144.2 (Tol), 144.6
(C3*), 142.6 (C8p), 130.6 (C5*), 129.6 (Tol), 129.3 (Tol), 129.2 (Tol), 129.2 (Tol), 126.6 (Tol), 125.8 (Tol), 122.6 (C5p), 108.9 (C4*), 86.1 (C11), 84.7 (C41), 74.9 (C31), 63.9 (C51), 38.4 (C21), 21.6 (2 ˆ CH
3) ppm.
ESI-MS m/z: [M + H]+539.2037 (calcd. 539.2043), [M + Na]+561.1857 (calcd. 561.1862). Elemental analysis (%): found: C 64.9, H 5.5, N 14.9; calcd. for C29H26N6O5¨0.2 C6H12: C 65.3, H 5.2, N 15.1.
4.5. 9-(2-Deoxy-β-D-erythro-pentofuranosyl)-6-(1H-pyrazol-1-yl)-9H-purine 3
A solution of aqueous NH3(25%, 30 mL) in CH3OH (30 mL) was added to compound 2 (0.200 g,
0.37 mmol). After stirring for 4 h, the solvent was removed in vacuo, and the residue was purified by silica gel column chromatography (cyclohexane (8):EtOAc (5):CH2Cl2(3):MeOH (1) Ñ cyclohexane
(8):EtOAc (5):CH2Cl2(3):MeOH (3)) to produce a brown foamy solid of the free nucleoside 3. Yield:
0.107 g (95%).1H NMR (400 MHz, D 2O, pD 7.2) δ: 8.64 (s, 1H, H5*), 8.57 (s, 1H, H2p), 8.55 (d, 1H, H3*), 7.94 (s, 1H, H8p), 6.66 (m, 1H, H4*), 6.49 (dd, 1H, H11), 4.69 (m, 1H, H31), 4.18 (m, 1H, H41), 3.85 (m, 2H, H51/H511), 2.83 (m, 1H, H21), 2.64 (m, 1H, H211) ppm.13C NMR (101 MHz, D 2O, pD 7.2), δ: 152.6 (C4p), 151.4 (C2p), 146.3 (C6p), 145.0 (C3*), 144.7 (C8p), 131.3 (C5*), 121.8 (C5p), 109.9 (C4*), 87.5 (C41),
84.8 (C11), 71.0 (C31), 61.5 (C51), 39.1 (C21) ppm. ESI-MS m/z: [M + Na]+325.1020 (calcd. 325.1025).
Elemental analysis (%): found: C 50.4, H 4.4, N 27.0; calcd. for C13H14N6O3¨0.5H2O: C 50.2, H 4.9,
N 27.0.
4.6. 9-[2-Deoxy-5-O-(4,41-dimethoxytriphenylmethyl)-β-D
-erythro-pentofuranosyl]-6-(1H-pyrazol-1-yl)-9H-purine 4
Compound 3 (106 mg, 0.334 mmol) was co-evaporated with dry pyridine (2 ˆ 20 mL) and dissolved in pyridine (10 mL) under argon atmosphere. After an addition of catalytic amounts of dimethylaminopyridine and 4,41-dimethoxytrityl chloride (193 mg, 0.568 mmol), the mixture was
stirred for 5 h. The solvent was removed in vacuo, and the crude material purified by silica gel column chromatography (CH2Cl2(100):MeOH (1) Ñ CH2Cl2(95):MeOH (5)). After recrystallization from
acetonitrile, product 4 was obtained as a white solid. Yield: 109 mg (54%).1H NMR (400 MHz, CDCl3),
δ: 9.05 (s, 1H, H5*), 8.73 (s, 1H, H2p), 8.29 (s, 1H, H8p), 7.96 (d, 1H, H3*) ,7.39 (m, 2H, DMT), 7.28 (m, 7H, DMT), 6.78 (m, 4H, DMT), 6.56 (m, 2H, H3*, H11), 4.71 (m, 1H, H31), 4.21 (m, 1H, H41), 3.75 (s, 6H, CH3(DMT)) 3.42 (m, 2H, H51/H511), 2.87 (m, 1H, H21), 2.62 (m, 1H, H211) ppm.13C NMR (101 MHz, CDCl3), δ: 158.5 (DMT), 153.3 (C4p), 152.0 (C2p), 147.2 (C6p), 144.5 (C3*), 144.4 (DMT), 143.1 (C8p), 135.6 (DMT), 130.9 (C5*), 130.0 (DMT), 128.1 (DMT), 127.9 (DMT), 126.9 (DMT), 122.7 (C5p), 113.1 (DMT), 109.0 (C4*), 86.6 (DMT), 86.3 (C41), 84.7 (C11), 72.3 (C31), 63.7 (C51), 55.3 (DMT), 40.5 (C21) ppm.
ESI-MS m/z: [M + Na]+627.2363 (calcd. 627.2326). Elemental analysis (%): found: C 67.3, H 5.7, N 14.4; calcd. for C34H32N6O5: C 67.5, H 5.3, N 13.9.
4.7. 9-[2-Deoxy-5-O-(4,41-dimethoxytriphenylmethyl)-β-D
-erythro-pentofuranosyl]-6-(1H-pyrazol-1-yl)-9H-purine 31-(2-cyanoethyl)-N,N1-diisopropyl phosphoramidite 5
The DMT-protected nucleoside 4 (162 mg, 0.268 mmol) was dissolved in dry CH2Cl2(3 mL)
under argon atmosphere. After adding N,N-diisopropylethylamine (0.238 mL, 0.113 mmol) and 2-cyanoethyl-N,N-diisopropylchlorophosporamidite (0.115 mL, 0.405 mmol), the mixture was stirred at ambient temperature for 2 h. The solvent was removed and the crude product purified by silica column chromatography (CH2Cl2(75):EtOAc (23):NEt3(2)). Yield 104 mg (48%).1H NMR (400 MHz,
CD3CN), δ: 9.12 (s, 1H, H5*), 8.69 (s, 1H, C2p), 8.40 (s, 1H, H8p), 7.90 (s, 1H, H3*), 7.36 (m, 2H, DMT), 7.23 (m, 7H, DMT), 6.75 (m, 4H, DMT), 6.63 (m, 1H, C4*), 6.51 (m, 1H, H11), 4.91 (m, 1H, H31), 4.25 (m, 1H, H41), 3.82 (m, 2H, O–CH 2), 3.71 (s, 6H, O–CH3), 3.62 (m, 2H, iPr-CH), 3.31 (m, 2H, H51, H511), 3.08 (m, 1H, H21), 2.65 (m, 3H, H211, CH 2–CN), 1.19 (m, 12H, iPr-CH3) ppm. 13C NMR (101 MHz, CD3CN), δ: 159.7 (DMT), 154,5 (C4p), 152.7 (C2p), 148.2 (C6p), 145.5 (C3*), 144.7 (C8p), 136.8 (DMT), 132.5 (C5*), 131.1 (DMT), 131.0 (DMT), 129.0 (DMT), 127.8 (DMT), 124.0 (C5p), 118.3 (CN), 114.0 (DMT), 110.0 (C4*), 87.1 (DMT), 86.4 (C41), 85.8 (C11), 73.9 (C31), 64.6 (C51), 59.5 (O–CH 2), 44.2 (iPr-CH), 39.3 (C21), 24.9 (iPr-CH 3), 21.1 (CH2–CN) ppm.31P NMR (162 MHz, CD3CN), δ: 148.2, 148.1 ppm.
Supplementary Materials: Supplementary materials can be found at http://www.mdpi.com/1422-0067/ 17/4/554/s1.
Acknowledgments:Financial support by the DFG (GRK 2027) and the NRW Graduate School of Chemistry is gratefully acknowledged.
Author Contributions:J. Christian Léon, Indranil Sinha and Jens Müller conceived and designed the experiments; J. Christian Léon and Indranil Sinha performed the experiments; J. Christian Léon and Jens Müller analyzed the data; Jens Müller wrote the paper.
Conflicts of Interest:The authors declare no conflict of interest. The founding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Abbreviations 6PP 6-Pyrazol-1-yl-purine A Adenine C Cytosine CD Circular dichroism G Guanine
MOPS 3-(N-Morpholino) propane sulfonate
T Thymine
References
1. Stulz, E.; Clever, G.H. DNA in Supramolecular Chemistry and Nanotechnology; John Wiley & Sons, Ltd.: Chichester, UK, 2015.
2. Müller, J. Metal-ion-mediated base pairs in nucleic acids. Eur. J. Inorg. Chem. 2008. [CrossRef]
3. Takezawa, Y.; Shionoya, M. Metal-mediated DNA base pairing: Alternatives to hydrogen-bonded Watson–Crick base pairs. Acc. Chem. Res. 2012, 45, 2066–2076. [CrossRef] [PubMed]
4. Tanaka, Y.; Kondo, J.; Sychrovský, V.; Šebera, J.; Dairaku, T.; Saneyoshi, H.; Urata, H.; Torigoe, H.; Ono, A. Structures, physicochemical properties, and applications of T–HgII–T, C–AgI–C, and other metallo-base-pairs. Chem. Commun. 2015, 51, 17343–17360. [CrossRef] [PubMed]
5. Clever, G.H.; Kaul, C.; Carell, T. DNA-metal base pairs. Angew. Chem. Int. Ed. 2007, 46, 6226–6236. [CrossRef]
[PubMed]
6. Johannsen, S.; Megger, N.; Böhme, D.; Sigel, R.K.O.; Müller, J. Solution structure of a DNA double helix with consecutive metal-mediated base pairs. Nat. Chem. 2010, 2, 229–234. [CrossRef] [PubMed]
7. Kumbhar, S.; Johannsen, S.; Sigel, R.K.O.; Waller, M.P.; Müller, J. A QM/MM refinement of an experimental DNA structure with metal-mediated base pairs. J. Inorg. Biochem. 2013, 127, 203–210. [CrossRef] [PubMed]
8. Kondo, J.; Yamada, T.; Hirose, C.; Okamoto, I.; Tanaka, Y.; Ono, A. Crystal structure of metallo-DNA duplex containing consecutive Watson–Crick-like T–HgII–T base pairs. Angew. Chem. Int. Ed. 2014, 53, 2385–2388.
[CrossRef] [PubMed]
9. Atwell, S.; Meggers, E.; Spraggon, G.; Schultz, P.G. Structure of a copper-mediated base pair in DNA. J. Am. Chem. Soc. 2001, 123, 12364–12367. [CrossRef] [PubMed]
10. Kondo, J.; Tada, Y.; Dairaku, T.; Saneyoshi, H.; Okamoto, I.; Tanaka, Y.; Ono, A. High-resolution crystal structure of a silver(I)–RNA hybrid duplex containing Watson–Crick-like C-silver(I)-C metallo-base pairs. Angew. Chem. Int. Ed. 2015, 54, 13323–13326. [CrossRef] [PubMed]
11. Johannsen, S.; Paulus, S.; Düpre, N.; Müller, J.; Sigel, R.K.O. Using in vitro transcription to construct scaffolds for one-dimensional arrays of mercuric ions. J. Inorg. Biochem. 2008, 102, 1141–1151. [CrossRef] [PubMed] 12. Scharf, P.; Müller, J. Nucleic acids with metal-mediated base pairs and their applications. ChemPlusChem
2013, 78, 20–34. [CrossRef]
13. Kaul, C.; Müller, M.; Wagner, M.; Schneider, S.; Carell, T. Reversible bond formation enables the replication and amplification of a crosslinking salen complex as an orthogonal base pair. Nat. Chem. 2011, 3, 794–800.
[CrossRef] [PubMed]
14. Liu, S.; Clever, G.H.; Takezawa, Y.; Kaneko, M.; Tanaka, K.; Guo, X.; Shionoya, M. Direct conductance measurement of individual metallo-DNA duplexes within single-molecule break junctions. Angew. Chem. Int. Ed. 2011, 50, 8886–8890. [CrossRef] [PubMed]
15. Ehrenschwender, T.; Schmucker, W.; Wellner, C.; Augenstein, T.; Carl, P.; Harmer, J.; Breher, F.; Wagenknecht, H.-A. Development of a metal-ion-mediated base pair for electron transfer in DNA. Chem. Eur. J. 2013, 19, 12547–12552. [CrossRef] [PubMed]
16. Léon, J.C.; Stegemann, L.; Peterlechner, M.; Litau, S.; Wilde, G.; Strassert, C.A.; Müller, J. Formation of silver nanoclusters from a DNA template containing Ag(I)-mediated base pairs. Bioinorg. Chem. Appl. 2016.
[CrossRef] [PubMed]
17. Taherpour, S.; Golubev, O.; Lönnberg, T. On the feasibility of recognition of nucleic acid sequences by metal-ion-carrying oligonucleotides. Inorg. Chim. Acta 2016. [CrossRef]
18. Taherpour, S.; Golubev, O.; Lönnberg, T. Metal-ion-mediated base pairing between natural nucleobases and bidentate 3,5-dimethylpyrazolyl-substituted purine ligands. J. Org. Chem. 2014, 79, 8990–8999. [CrossRef]
[PubMed]
19. Taherpour, S.; Lönnberg, H.; Lönnberg, T. 2,6-Bis(functionalized) purines as metal-ion-binding surrogate nucleobases that enhance hybridization with unmodified 21-O-methyl oligoribonucleotides. Org. Biomol. Chem. 2013, 11, 991–1000. [CrossRef] [PubMed]
20. Taherpour, S.; Lönnberg, H. Metal ion chelates as surrogates of nucleobases for the recognition of nucleic acid sequences: The Pd2+complex of 2,6-bis(3,5-dimethylpyrazol-1-yl)purine riboside. J. Nucleic Acids 2012.
[CrossRef] [PubMed]
21. Golubev, O.; Lönnberg, T.; Lönnberg, H. Metal-ion-binding analogs of ribonucleosides: Preparation and formation of ternary Pd2+and Hg2+complexes with natural pyrimidine nucleosides. Helv. Chim. Acta 2013, 96, 1658–1669. [CrossRef]
22. Sinha, I.; Hepp, A.; Kösters, J.; Müller, J. Metal complexes of 6-pyrazolylpurine derivatives as models for metal-mediated base pairs. J. Inorg. Biochem. 2015, 153, 355–360. [CrossRef] [PubMed]
23. Estep, K.G.; Josef, K.A.; Bacon, E.R.; Carabateas, P.M.; Rumney, S., IV; Pilling, G.M.; Krafte, D.S.; Volberg, W.A.; Dillon, K.; Dugrenier, N.; et al. Synthesis and Structure-Activity Relationships of 6-Heterocyclic-Substituted Purines as Inactivation Modifiers of Cardiac Sodium Channels. J. Med. Chem. 1995, 38, 2582–2595. [CrossRef]
[PubMed]
24. Scharf, P.; Jash, B.; Kuriappan, J.A.; Waller, M.P.; Müller, J. Sequence-dependent duplex stabilization upon formation of a metal-mediated base pair. Chem. Eur. J. 2016, 22, 295–301. [CrossRef] [PubMed]
25. Richters, T.; Krug, O.; Kösters, J.; Hepp, A.; Müller, J. A family of “click” nucleosides for metal-mediated base pairing: Unravelling the principles of highly stabilising metal-mediated base pairs. Chem. Eur. J. 2014, 20, 7811–7818. [CrossRef] [PubMed]
26. Schweizer, K.; Kösters, J.; Müller, J. 4-(21-Pyridyl)imidazole as an artificial nucleobase in highly stabilizing Ag(I)-mediated base pairs. J. Biol. Inorg. Chem. 2015, 20, 895–903. [CrossRef] [PubMed]
27. Litau, S.; Müller, J. Mononuclear 1,3-dideazaadenine-Ag(I)-thyminate base pairs. Z. Anorg. Allg. Chem. 2015, 641, 2169–2173. [CrossRef]
28. Sinha, I.; Kösters, J.; Hepp, A.; Müller, J. 6-Substituted purines containing thienyl or furyl substituents as artificial nucleobases for metal-mediated base pairing. Dalton Trans. 2013, 42, 16080–16089. [CrossRef]
[PubMed]
29. Mandal, S.; Hepp, A.; Müller, J. Unprecedented dinuclear silver(I)-mediated base pair involving the DNA lesion 1,N6-ethenoadenine. Dalton Trans. 2015, 44, 3540–3543. [CrossRef] [PubMed]
30. Switzer, C.; Sinha, S.; Kim, P.H.; Heuberger, B.D. A purine-like nickel(II) base pair for DNA. Angew. Chem. Int. Ed. 2005, 44, 1529–1532. [CrossRef] [PubMed]
31. Kim, E.-K.; Switzer, C. Bis(6-carboxypurine)-Cu2+: A possibly primitive metal-mediated nucleobase pair. Org. Lett. 2014, 16, 4059–4061. [CrossRef] [PubMed]
32. Swasey, S.M.; Espinosa Leal, L.; Lopez-Acevedo, O.; Pavlovich, J.; Gwinn, E.G. Silver(I) as DNA glue: Ag+-mediated guanine pairing revealed by removing Watson–Crick constraints. Sci. Rep. 2015, 5, 10163.
[CrossRef] [PubMed]
33. Richters, T.; Müller, J. A metal-mediated base pair with a [2+1] coordination environment. Eur. J. Inorg. Chem. 2014. [CrossRef]
34. Sinha, I.; Fonseca Guerra, C.; Müller, J. A highly stabilizing silver(I)-mediated base pair in parallel-stranded DNA. Angew. Chem. Int. Ed. 2015, 54, 3603–3606. [CrossRef] [PubMed]
35. Heuberger, B.D.; Shin, D.; Switzer, C. Two Watson–Crick-like metallo base-pairs. Org. Lett. 2008, 10, 1091–1094. [CrossRef] [PubMed]
36. Mei, H.; Röhl, I.; Seela, F. Ag+-mediated DNA base pairing: Extraordinarily stable pyrrolo-dC–pyrrolo-dC pairs binding two silver ions. J. Org. Chem. 2013, 78, 9457–9463. [CrossRef] [PubMed]
37. Jana, S.K.; Guo, X.; Mei, H.; Seela, F. Robust silver-mediated imidazolo-dC base pairs in metal DNA: Dinuclear silver bridges with exceptional stability in double helices with parallel and antiparallel strand orientation. Chem. Commun. 2015, 51, 17301–17304. [CrossRef] [PubMed]
38. Rolland, V.; Kotera, M.; Lhomme, J. Convenient preparation of 2-deoxy-3,5-di-O-p-toluoyl-α-D -erythro-pentofuranosyl chloride. Synth. Commun. 1997, 27, 3505–3511. [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).