Vascular Smooth Muscle Cells Secrete CXCL10 in Response to Heat Shock Protein 90
전체 글
수치
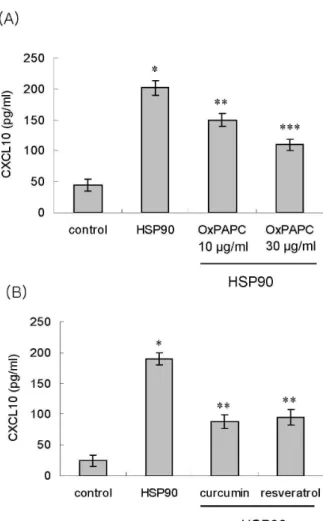
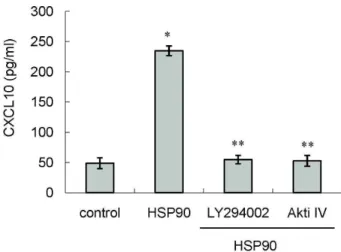
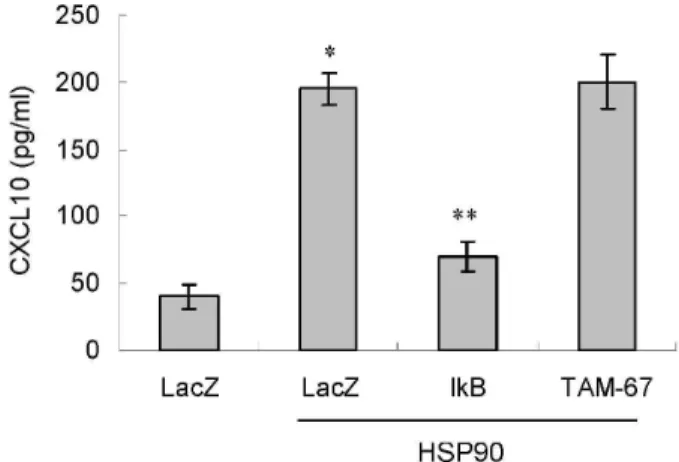
관련 문서
Conclusion: In conclusion, the relaxant effects of neuromuscular blockers on the uterine smooth muscle may be transmitted via nicotinic acetylcholine receptors
whether NME1-depleted cells impairs the Rad51 foci formation in response
We found that FoxO1 is constantly increased in MCF-7/ADR, adriamycin-resistant breast cancer cells, and FoxO1 has a critical role in the MDR1 gene expression (16).. There is
In this study, we found that Pin1 plays a pivotal role in insulin- induced AP-1 activation through its interaction with p70S6K and prolongs activity of
In the current study, I investigated the association between vascular risk factors as chronic ischemia of the prostate and BPH by conducting experiments to elucidate
• Generation of different specialized kinds of cells from zygote (fertilized egg) or other precursor cells.. – Generate blood cells, muscle
The improvement factors of the performance showed significant increase in muscle strength, muscle endurance, cardiovascular endurance, power, agility and
In the health fitness category of the adult women who underwent instrument pilates exercise, they were statistically significant in muscle strength,