6.9세 다운증후군 소아에서 발생한 GATA1 유전자변이를 동반한 급성거핵모구백혈병
박주현1ㆍ이윤경1ㆍ박미림1ㆍ손보라2ㆍ서자영3ㆍ김희진3ㆍ임호준4ㆍ임연정5
충북대학교 의과대학 1소아청소년과학교실, 2진단검사의학교실, 3성균관대학교 의과대학 삼성서울병원 진단검사의학교실,
4울산대학교 의과대학 서울아산병원 소아청소년과학교실, 5충남대학교 의과대학 소아청소년과학교실
GATA1-positive Acute Megakaryoblastic Leukemia in a 6.9-year-old Patient with Down Syndrome: What is the Prognosis?
Joohyun Park, M.D.1, Youn Kyung Lee, M.D.1, Meerim Park, M.D.1, Bo Ra Son, M.D.2, Ja Young Seo, M.D.3, Hee Jin Kim, M.D.3, Ho Joon Im, M.D.4 and Yeon Jung Lim, M.D.5
Departments of 1Pediatrics, 2Laboratory Medicine, Chungbuk National University College of Medicine, Cheongju,
3Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul,
4Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul,
5Department of Pediatrics, Chungnam National University College of Medicine, Daejeon, Korea
We describe a very rare case of 6.9-year-old boy with Down syndrome (DS) and a prior history of transient myeloproliferative disorder. He was diagnosed with acute mega- karyoblastic leukemia and found to have a novel GATA1 gene mutation, as well as a complex karyotype without recurrent acute myeloid leukemia (AML) aberrations. The patient achieved an early bone marrow response to chemotherapy. However, relapse occurred during treatment, 9 months after the initial diagnosis. Although GATA1 muta- tions are closely associated with DS-AML, we speculate that factors other than the pres- ence of the GATA1 mutation can affect the overall outcome in older pediatric patients.
pISSN 2233-5250 / eISSN 2233-4580 Clin Pediatr Hematol Oncol 2013;20:66∼70
Received on March 29, 2013 Revised on April 16, 2013 Accepted on April 20, 2013
Corresponding author: Meerim Park Department of Pediatrics, Chungbuk National University College of Medicine, 1473, Seobu-ro, Heungdeok-gu, Cheongju 361-240, Korea Tel: +82-43-269-6338 Fax: +82-43-264-6620 E-mail: ming2a@daum.net
Key Words: Down syndrome, Acute megakaryoblastic leukemia, Older, GATA1
Introduction
Children with Down syndrome (DS) have a significantly increased risk of developing acute myeloid leukemia (AML), typically acute megakaryoblastic leukemia (AMKL),
subtype M7 in the French-American-British (FAB) classi- fication system [1]. DS-AML is usually characterized by a mutation in the hematopoietic transcription factor GATA1.
Presence of the GATA1 mutation contributes to high intra-
cellular accumulation of cytosine arabinoside (ara-C) and
confers as an excellent outcome in patients with DS-AML
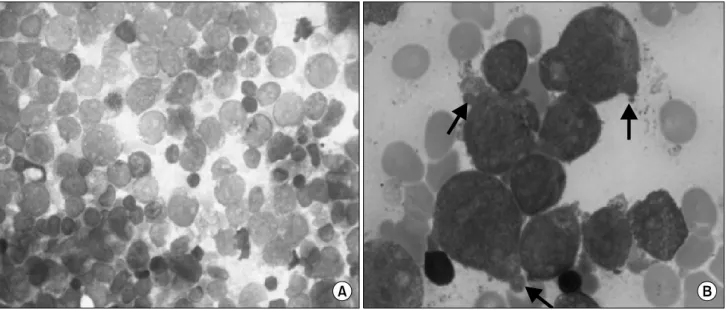
Fig. 1. Bone marrow smear at the time of diagnosis. (A) Magnification ×400, Wright-Giemsa staining, (B) Cytoplasmic blebs (arrows) are demonstrated on the surface of several blasts. Magnification ×1,000, Wright-Giemsa staining.
[2,3]. Hasle et al. [4] reported that children aged ≥ 4 years with DS-AML generally do not have GATA1 mutations and their disease behaves in a similar fashion to sporadic AML.
They propose that GATA1 mutations may be a better mark- er than age, blast type, or FAB type in predicting outcomes in DS-AML patients.
The age distribution of DS-AML is very distinctive, with about 50% of the patients diagnosed at 1 year of age, about one-third at 2 years of age, and only 1-2% diagnosed at more than 4 years of age [5]. Clinical trial data suggest that, although DS-AML generally has very favorable outcomes, older patients may have inferior survival [6,7]. However, no large studies of the genetic prognostic factors associated with older pediatric DS-AML have been conducted, mainly due to the very low incidence of AML among older DS children. There are very few data describing the clinical course of older DS-AML patients with the GATA1 mutation [8]; neither the prevalence nor the prognosis of the GATA1 mutation in older DS-AML patients is yet known.
We describe a very rare case: a 6.9-year-old boy with DS-AML found to have a novel GATA1 gene mutation and complex cytogenetic aberrations.
Case Report
A 6.9-year-old boy with DS was admitted to our in- stitution because of petechiae over his entire body. The pa- tient had a history of transient myeloproliferative disorder (TMD) at age of one month. At that time, he received low dose ara-C treatment for 7 days and his blood count recov- ered to the normal range.
At the time of his admission at age 6.9 years, his physical
examination revealed abdominal distension with hep-
atosplenomegaly, and multiple petechiae. A complete blood
cell count showed a hemoglobin concentration of 10.4 g/dL,
a platelet count of 6,000 /μL, and white blood cell count
of 10,040 /μL with 48% segmented neutrophils, 33% lympho-
cytes, and 18% blasts. On the peripheral blood smear, a
number of cells with deep basophilic staining, suspicious
for blasts, were noted. Bone marrow (BM) examination re-
vealed malignant myeloid cells with cytoplasmic blebs, con-
sistent with megakaryoblasts (Fig. 1). Flow cytometric analy-
sis revealed that these blasts were positive for CD7, CD13,
CD34, CD41, CD56, and CD117. Immunocytochemical stain-
ing was negative for peroxidase and Sudan Black B. The
diagnosis of AMKL was made according to the morpho-
logical and immunophenotypic criteria of the FAB classi-
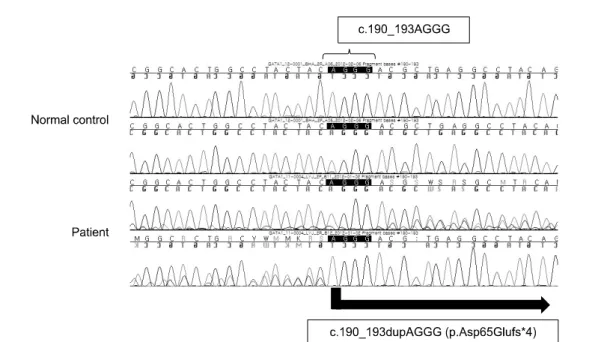
Fig. 2. Direct sequencing analysis of the GATA1 gene. Mutation, c.190_193dupAGGG (p.Asp65Gluf*4), is identified in the N-terminal activation domain of GATA1 exon 2.
fication system. Cerebrospinal fluid examination was normal.
Cytogenetic analysis of the BM cells revealed 46, XX, del (6)(q13q23), der(7;15)(q10;q10), del(9)(p22), +14, der(14;21) (q10;q10)×2, +21, +21 [13] / 46, XY, der(14;21), +21 [7].
The patient’s constitutional karyotype was 46, XY, +21, der (14;21)(q10;q10). Genomic DNA was isolated from BM aspi- rates and GATA1 analysis was performed with reverse tran- scription polymerase chain reaction followed by direct se- quencing [9]. A mutation not previously reported for AMKL, c.190_193dupAGGG (p.Asp65Gluf*4), was identified in exon 2 of GATA1, introducing a premature stop codon after amino acid 68 (Fig. 2). Molecular analysis of the FLT3, MLL and RUNX1 genes did not detect any alterations.
The patient was started on anti-leukemic chemotherapy according to the Children’s Oncology Group protocol for AML in DS (COG A2971). On day 14 of induction chemo- therapy, BM examination showed 4% myeloblasts with 10%
cellularity; it is reported that approximately 74% of patients with DS showed morphologic remission in COG A2971 trial [10]. The patient achieved complete morphologic remission along with cytogenetic remission at the end of induction phase therapy. However, the patient repeatedly suffered from severe infections during the course of chemotherapy, including an invasive fungal infection of the lung, infective
endocarditis, a catheter-related infection, and a brain abscess.
Furthermore, he experienced grade ≥2 liver toxicity, as well as renal toxicity. Scheduled chemotherapy was occa- sionally interrupted either for infection or for prolonged myelosuppression. Nine months after the initial diagnosis, a BM relapse occurred during intensification therapy.
Although salvage chemotherapy with fludarabine and ara-C was given, the patient did not achieve remission and died of septic shock 3 months after the relapse.
Discussion
DS-AML is usually characterized by a mutation in the GATA1 gene on X chromosome that leads to expression of N-terminally truncated GATA1 protein (GATA1s). Most reported mutations, including insertions, deletions, and point mutations, are found in exon2 of GATA1 [9]. In pa- tients who progress from TMD to DS-AML, blast analysis shows the same GATA1 mutation in both conditions, dem- onstrating the clonal relationship of these disease states [11,12]. Recently, Alford et al. [9] found that the specific type of GATA1 mutation does not predict which patients with TMD will progress to DS-AML.
Somatic mutations within exon 2 of GATA1 result in the
differential regulation of target genes. One of these targets is the gene encoding the enzyme cytidine deaminase (CDA), which catalyzes the deamination of ara-C into its inactive derivative [2,3]. GATA1 mutations in DS blasts cause a decrease in the transcription and expression of the CDA gene, thus decreasing the inactivation of ara-C, and subsequently causing an increase in its cytotoxic efficacy.
Zwann et al. [13] demonstrated a 12-fold increase in sensi- tivity to ara-C in patients with DS-AML over those with AML who do not have DS. These findings would explain the fa- vorable prognosis in DS-AML patients with GATA1 mutation [13,14]. The GATA1 mutation and the resultant short-form GATA1s protein may be associated with changes in the transactivation activity of many genes that influence treat- ment response, not only CDA [15,16]. Therefore, the identi- fication of GATA1 mutations in patients with DS-AML is crit- ical to the understanding of both the mechanism of leuke- mogenesis in DS and the very high sensitivity of these cells to cytotoxic agents [17].
Clinical trials have suggested that older children with DS and AMKL have inferior outcomes compared with children younger than 4 years of age, primarily a result of poor re- mission induction rather than chemotherapeutic toxicity [6].
It has also been shown that older children with DS often lack the GATA1 mutation, and that the biological behavior of the leukemia present in these children is similar to that seen in the sporadic AML in children without DS [4]. This finding was confirmed in the COG A2971 trial: the authors concluded that older patients with DS may require more intensive therapy, such as that given to AML patients with- out DS [10].
Our patient, an almost-7-year-old child with AMKL and a GATA1 mutation, is therefore quite rare. The particular GATA1 mutation detected in our patient has not been pre- viously identified in the reported DS-AML patient series.
Our patient had a clinical history of TMD, but unfortunately we did not confirm the presence of the GATA1 mutation at that time; congenital GATA1 mutations may be present in many DS patients who later develop AMKL [12]. Despite the presence of the GATA1 mutation and the administration of standard DS-AML therapy, our patient experienced early relapse. He frequently suffered from severe infections dur-
ing the period of myelosuppression, compromising his chemotherapy. Moreover, dose-intensity of COG A2971 chemotherapy caused him significant toxicity. Increased toxic chemotherapy complications in patients with DS have been reported by several investigators, and are presumed to be caused by increased chemosensitivity [18,19]. Yet in terms of treatment outcomes, our patient did not respond in the way predicted by his disease status.
Although DS-AML in older pediatric patients is generally considered to be similar to de novo AML, our patient had the GATA1 mutation and a prior history of TMD, suggesting that his leukemogenesis was similar to that of typical DS-AML patients <4 years of age. Although the GATA1 mutations are closely associated with AML in DS patients, mostly in younger children and conferring a good prog- nosis, we speculate that other factors can affect the overall outcome in patients with DS-AML irrespective of the pres- ence of GATA1 mutation. Having additional chromosomal aberrations may have been a poor prognostic factor for our patient. That is to say, although our patient was affected with the unique DS-AML characterized by the GATA1 muta- tion, his complex karyotype abnormality could have abro- gated the favorable clinical course usually seen with that disease. Whether older age can affect a different behavior in leukemic cell biology (e.g., in the GATA1 mutation) re- mains to be determined. Other mechanisms may also be involved in determining the chemotherapy sensitivity of DS megakaryoblasts. Over 500 genes have been found to be differentially expressed in DS and non-DS megakaryoblasts [20]; some of these genes may also have contributed to the poor outcome seen in our patient.
Our observation suggests that DS-AML in older children may follow a poor clinical course, even in the presence of a GATA1 mutation. Because the prognostic factors for DS-AML are still unknown, particularly in patients over 4 years of age, more data accumulation is needed before the GATA1 mutation can be used as a surrogate marker for DS-AML prognosis.
Acknowledgements
We wish to thank our patient and his family for facilitat-
ing this report.
References
1. Lange B. The management of neoplastic disorders of haema- topoiesis in children with Down's syndrome. Br J Haematol 2000;110:512-24.
2. Ge Y, Jensen TL, Stout ML, et al. The role of cytidine deami- nase and GATA1 mutations in the increased cytosine arabino- side sensitivity of Down syndrome myeloblasts and leukemia cell lines. Cancer Res 2004;64:728-35.
3. Ge Y, Stout ML, Tatman DA, et al. GATA1, cytidine deami- nase, and the high cure rate of Down syndrome children with acute megakaryocytic leukemia. J Natl Cancer Inst 2005;97:
226-31.
4. Hasle H, Abrahamsson J, Arola M, et al. Myeloid leukemia in children 4 years or older with Down syndrome often lacks GATA1 mutation and cytogenetics and risk of relapse are more akin to sporadic AML. Leukemia 2008;22:1428-30.
5. Hasle H. Pattern of malignant disorders in individuals with Down's syndrome. Lancet Oncol 2001;2:429-36.
6. Gamis AS, Woods WG, Alonzo TA, et al. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia:
a report from the Children's Cancer Group Study 2891. J Clin Oncol 2003;21:3415-22.
7. Creutzig U, Reinhardt D, Diekamp S, Dworzak M, Stary J, Zimmermann M. AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia 2005;19:1355-60.
8. Ariffin H, Garcia JC, Daud SS, et al. GATA1 mutations in pa- tients with down syndrome and acute megakaryoblastic leu- kaemia do not always confer a good prognosis. Pediatr Blood Cancer 2009;53:108-11.
9. Alford KA, Reinhardt K, Garnett C, et al. Analysis of GATA1 mutations in Down syndrome transient myeloproliferative dis-
order and myeloid leukemia. Blood 2011;118:2222-38.
10. Sorrell AD, Alonzo TA, Hilden JM, et al. Favorable survival maintained in children who have myeloid leukemia associated with Down syndrome using reduced-dose chemotherapy on Children's Oncology Group trial A2971: a report from the Children's Oncology Group. Cancer 2012;118:4806-14.
11. Klusmann JH, Creutzig U, Zimmermann M, et al. Treatment and prognostic impact of transient leukemia in neonates with Down syndrome. Blood 2008;111:2991-8.
12. Ahmed M, Sternberg A, Hall G, et al. Natural history of GATA1 mutations in Down syndrome. Blood 2004;103:2480-9.
13. Zwaan CM, Kaspers GJ, Pieters R, et al. Different drug sensi- tivity profiles of acute myeloid and lymphoblastic leukemia and normal peripheral blood mononuclear cells in children with and without Down syndrome. Blood 2002;99:245-51.
14. Gurbuxani S, Vyas P, Crispino JD. Recent insights into the mechanisms of myeloid leukemogenesis in Down syndrome.
Blood 2004;103:399-406.
15. Zipursky A, Peeters M, Poon A. Megakaryoblastic leukemia and Down's syndrome: a review. Pediatr Hematol Oncol 1987;4:211-30.
16. Hitzler JK, Cheung J, Li Y, Scherer SW, Zipursky A. GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood 2003;101:4301-4.
17. Wechsler J, Greene M, McDevitt MA, et al. Acquired muta- tions in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet 2002;32:148-52.
18. Creutzig U, Ritter J, Vormoor J, et al. Myelodysplasia and acute myelogenous leukemia in Down's syndrome. A report of 40 children of the AML-BFM Study Group. Leukemia 1996;
10:1677-86.
19. Levitt GA, Stiller CA, Chessells JM. Prognosis of Down's syn- drome with acute leukaemia. Arch Dis Child 1990;65:212-6.
20. Ge Y, Dombkowski AA, LaFiura KM, et al. Differential gene expression, GATA1 target genes, and the chemotherapy sensi- tivity of Down syndrome megakaryocytic leukemia. Blood 2006;107:1570-81.