증례7 증례5
증례8 증례6
증례9 종설1 원저1 원저2
증례1 원저3
증례2 증례3 증례4
연세대학교 의과대학 강남세브란스병원 신경외과학교실 박세준, 조용은, 박현호, 이규성
Department of Neurosurgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
Se-Jun Park, Yong-Eun Cho, Hun Ho Park, Kyu-Sung Lee
두개강내 투명세포성 수막종 수술 및 방사선 치료 후 척추내 원위 재발: 증례보고 및 문헌 고찰
J Korean Skull Base Society 15권 2호 : 109~115, 2020
Clear cell meningioma (CCM) is a rare subtype of World Health Organization grade II meningioma with a high rate of recurrence. Only a limited number of cases of distal recurrence or metastasis of CCM have been reported. We report a rare case of distal CCM relapse from an intracranial to intraspinal lesion. A 29-year-old female presented with diplopia and right partial sixth nerve palsy. Brain magnetic resonance imaging (MRI) revealed petroclival meningioma extending to the middle cranial fossa and right cavernous sinus. The tumor was subtotally resected and pathologically confirmed to be CCM. The patient underwent adjuvant radiotherapy targeting the remnant tumor site and intracranial tumor bed. At follow- up, MRI revealed a stable disease. Six years later, the patient developed lower back pain and bilateral buttock pain. Spine MRI revealed multiple intradural extramedullary tumors at the L2, L3, and L4-5 level, with the largest at the L3 level. The tumor at the L3 level was grossly totally resected in an en bloc manner. Histopathological findings revealed CCM. The patient underwent additional radiotherapy targeting the spinal canal and residual intraspinal tumors.
CCMs can recur distally or metastasize throughout the whole neuraxis. Therefore, evaluation of the whole neuraxis is recommended.
Distal recurrence of intraspinal clear cell meningioma after intracranial surgery and adjuvant radiotherapy: A case report
and literature review
논문 접수일 : 2020년 6월 7일 논문 완료일 : 2020년 7월 16일 주소 : Department of Neurosurgery,
Gangnam Severance Hospital, Spine and Spinal Cord Institute, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul 06273, Korea Tel : +82-2-2019-3390 Fax : +82-2-3461-9229 E-mail : yecho@yuhs.ac
교신저자
Clear cell meningioma, Metastasis, Recurrence Key Words
Yong-Eun Cho
▒ INTRODUCTION
Clear cell meningioma (CCM) is a rare histological
type of meningioma that is classified as a World Health Organization (WHO) grade II tumor of the nervous system.
[1] It has been reported that CCM usually occurs in
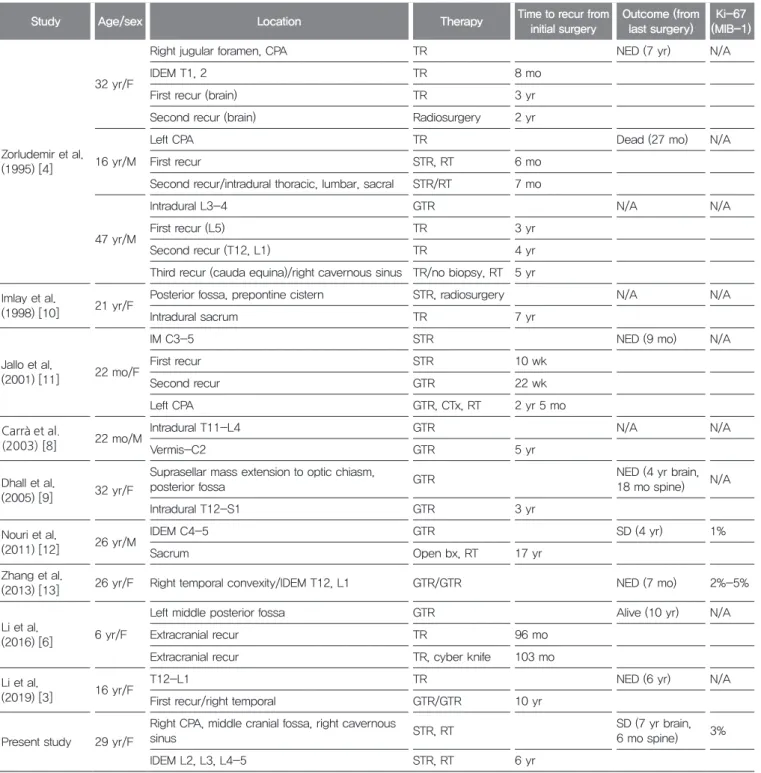
Table 1. Characteristics of included studies
Study Age/sex Location Therapy Time to recur from
initial surgery
Outcome (from last surgery)
Ki-67 (MIB-1)
Zorludemir et al.
(1995) [4]
32 yr/F
Right jugular foramen, CPA TR NED (7 yr) N/A
IDEM T1, 2 TR 8 mo
First recur (brain) TR 3 yr
Second recur (brain) Radiosurgery 2 yr
16 yr/M
Left CPA TR Dead (27 mo) N/A
First recur STR, RT 6 mo
Second recur/intradural thoracic, lumbar, sacral STR/RT 7 mo
47 yr/M
Intradural L3-4 GTR N/A N/A
First recur (L5) TR 3 yr
Second recur (T12, L1) TR 4 yr
Third recur (cauda equina)/right cavernous sinus TR/no biopsy, RT 5 yr Imlay et al.
(1998) [10] 21 yr/F Posterior fossa, prepontine cistern STR, radiosurgery N/A N/A
Intradural sacrum TR 7 yr
Jallo et al.
(2001) [11] 22 mo/F
IM C3-5 STR NED (9 mo) N/A
First recur STR 10 wk
Second recur GTR 22 wk
Left CPA GTR, CTx, RT 2 yr 5 mo
Carrà et al.
(2003) [8] 22 mo/M Intradural T11-L4 GTR N/A N/A
Vermis-C2 GTR 5 yr
Dhall et al.
(2005) [9] 32 yr/F
Suprasellar mass extension to optic chiasm,
posterior fossa GTR NED (4 yr brain,
18 mo spine) N/A
Intradural T12-S1 GTR 3 yr
Nouri et al.
(2011) [12] 26 yr/M IDEM C4-5 GTR SD (4 yr) 1%
Sacrum Open bx, RT 17 yr
Zhang et al.
(2013) [13] 26 yr/F Right temporal convexity/IDEM T12, L1 GTR/GTR NED (7 mo) 2%-5%
Li et al.
(2016) [6] 6 yr/F
Left middle posterior fossa GTR Alive (10 yr) N/A
Extracranial recur TR 96 mo
Extracranial recur TR, cyber knife 103 mo
Li et al.
(2019) [3] 16 yr/F T12-L1 TR NED (6 yr) N/A
First recur/right temporal GTR/GTR 10 yr
Present study 29 yr/F
Right CPA, middle cranial fossa, right cavernous
sinus STR, RT SD (7 yr brain,
6 mo spine) 3%
IDEM L2, L3, L4-5 STR, RT 6 yr
F: female, M: male, CPA: cerebellopontine angle, IDEM: intradural extramedullary, recur: recurrence, IM: intramedullary, TR: tumor removal (gross total/subtotal resection unknown), STR: subtotal resection, RT: radiotherapy, GTR: gross total removal, CTx: Chemotherapy, bx: biopsy, NED: no evidence of disease, N/A: not available, SD: stable disease.
younger patients, recurs in approximately 42% to 60%
of cases, and metastasizes in up to 4.1% of cases.[2-6]
Approximately 0.1% to 0.2% of meningioma cases have been reported to demonstrate extracranial meningioma,[7]
and only a few cases of distal recurrence and metastasis of CCM have been reported.[3,4,6,8-13] We report a rare case of distal recurrence of intraspinal CCM after intracranial surgery and adjuvant radiotherapy. To the author’s knowledge, only 11 cases of distal recurrence or metastasis of CCMs have been reported,[3,4,6,8-13]
which are also presented in Table 1.
▒ CASE REPORT
A 29-year-old female presented with a 3-month history of diplopia and partial right 6th nerve palsy that had worsened over time. No other neurologic deficit was present upon admission. T2-weighted magnetic resonance imaging (MRI) showed an extra-axial mass measuring 5.2 × 3.9
× 3.8 cm in size with heterogenous enhancement in the right cerebellopontine angle cistern and prepontine cistern extending to the middle cranial fossa and right cavernous sinus (Fig. 1). She underwent combined petrosal approach craniotomy and tumor removal. The tumor was a relatively well defined, soft, and mixed yellow-white-red mass. The
tumor was grossly subtotally resected. Tissue pathology confirmed the diagnosis of CCM; the tumor was positive for epithelial membrane antigen epithelial membrane antigen (EMA) and Periodic acid-Schiff (PAS) and negative for S-100 protein, and the Ki-67 index was 3%. Postoperative MRI showed minimal residual tumor in the right cavernous sinus and petrous apex (Fig. 2). Adjuvant radiotherapy was performed one month after initial surgery, targeting the remnant tumor site, tumor bed (54 Gy/27 fraction [fx]), and margin (47.25 Gy/27 fx). At follow-up, approximately 56 months after radiotherapy, MRI showed stable disease with no significant interval change of the remnant tumor in the intracranial area (Fig. 3).
Six years after the initial intracranial operation, the patient complained of newly developed lower back pain and bilateral buttock pain that had been present for 5 months, with the symptoms worsening over time. Lumbar MRI revealed at least 4 intradural extramedullary tumors at the L2, L3, and L4-5 levels, with the largest tumor at the L3 level measuring 2.3 × 1.1 cm, splitting the cauda equina (Fig. 4). Surgery was performed on the largest tumor at the L3 level with a presumptive diagnosis of neurogenic tumor. The tumor was firm, white, and poor in vascularity, and the minimal adhesion to the rootlet was dissected with ease. The tumor at L3 level was grossly Fig. 1
A B C
Preoperative intracranial magnetic resonance imaging.
(A) T1-enhance axial image. (B) T1-enhance sagittal image. (C) T1-enhance coronal image.
totally resected in an en bloc manner (Fig. 5). Histologic findings confirmed the diagnosis of CCM; the tumor was positive for PAS and EMA and negative for D-PAS and HMB45, and the Ki-67 index was 3%. Her symptoms were relieved after surgery. Adjuvant radiotherapy targeting the tumor bed (45 Gy/20 fx), gross residual tumor at L2 and L4 (48 Gy/20 fx), and L1 to S1 spinal canal and margin (43 Gy/20 fx) was performed two months after the surgery.
The follow-up MRI image was taken three months after adjuvant radiotherapy on intraspinal lesions and proved no significant difference compared with postoperative
MRI images. At last follow-up which is seven year from initial intracranial operation and one year from intraspinal operation, both intracranial and intraspinal remnant lesions were regarded as stable disease.
▒ DISCUSSION
CCM is a subtype of WHO grade II meningioma characterized by the histologically unique feature of polygonal cells with clear glycogen-rich cytoplasm.[4] It accounts for approximately 0.3% of all meningiomas.[5,6]
Fig. 2
A B C
Postoperative intracranial magnetic resonance imaging (MRI). MRI one month after surgery showing remnant tumor.
(A) T1-enhance axial image. (B) T1-enhance sagittal image. (C) T1-enhance coronal image.
Fig. 3
A B C
Post-adjuvant radiotherapy intracranial magnetic resonance imaging (MRI). MRI five months after initial surgery, which is three months after adjuvant radiotherapy, showing no significant difference in tumor size from postoperative MRI images.
(A) T1-enhance axial image. (B) T1-enhance sagittal image. (C) T1-enhance coronal image.
It is known for its aggressive behavior despite its benign pathology with a high rate of recurrence of up to 60%
[4] and 38% for spinal CCMs.[3] About 4.1% of CCMs are known for distal recurrence and metastasis.[2-5] Singh et al. [7] described that meningiomas with extracranial metastases, usually to the lung, liver, lymph nodes, long bones, and spinal vertebrae, occur in approximately 0.1%
to 0.2% of all meningiomas. Due to its histopathologically rare nature, only a few case reports on distal recurrence and metastasis of CCMs have been reported. This is the first case with distal metastasis in a CCM patient to be published in Korea.
There have been a total of 5 reported cases of intracranial CCM with postoperative discovery of intraspinal CCM, including our case,[4,9,10] 4 patients with intraspinal CCM followed by intracranial CCM,[3,4,8,11]
1 patient with simultaneous intracranial and intraspinal CCM,[13] 1 patient with distal recurrence of an intraspinal lesion,[12] and 1 patient with an unspecified extracranial metastasis.[6] It is unclear whether these presentations should be regarded as distal recurrence, metastasis, or multifocal synchronous incidence because like in our case, most cases did not involve full investigation of the whole neuraxis at the initial evaluation. The pathophysiology of CCM metastasis is unknown; however, hematogenous,
lymphatic, cerebrospinal fluid and perioperative tumor seeding have been proposed as possible routes.[7] Lee et al. [14] reported a patient with diffuse leptomeningeal seeding after surgical resection of an intracranial CCM.
Singh et al. [7] suggested that vertebral metastasis may have occurred via a hematogenous pathway. Due to these reasons, evaluation of the whole neuraxis is necessary, and systematic assessment of other organs, such as the lung, liver, and lymph node, may also be needed.[7]
Previous craniotomy, venous sinus invasion, local recurrence, histological malignancy, hypercellularity, cellular heterogeneity, high mitotic activity, nuclear pleomorphism, and necrosis are known risk factors for extracranial metastasis of meningioma.[7] Subtotal resection of the tumor, male sex, and no adjuvant postoperative radiotherapy have been mentioned by Zhang et al. [2] as possible predictors of tumor recurrence in intracranial CCMs. It is still controversial if immunohistochemical markers, such as Ki-67, p53 labeling index, and CD90, are related to recurrence.
[2,4,6,15] In our case the patient had multiple risk factors including previous craniotomy, right cavernous sinus invasion, and subtotal resection of the intracranial CCM.
Zorludemir et al. [4] inferred that CCMs may be related to neurofibromatosis type 2, which can present with multiple
Fig. 4
A B C D
Preoperative intraspinal magnetic resonance imaging.
(A) T2 sagittal image. (B) T2 sagittal image. (C) T1- enhance sagittal image. (D) T1-enhance axial image at L3 level.
meningiomas.
The mean age at diagnosis of CCM was 29 to 32 years,[5,6] relatively younger than that of all meningiomas. In a review of cases, the average age of patients with CCM with distal recurrence or metastasis was between 22 months and 47 years, with a median age of 23.5 years. Yet, its clinical significance is uncertain whether these patients tend to have higher proliferation that it gets detected earlier in age.
Li et al. [6] stated that the time for CCM to recur varied from 10 months to 12 years with a median time of 29 months, while Singh et al. [7] reported 3 months to 14 years with a median time of 78 months for extracranial meningioma metastasis. Following a review of literature, we identified 11 patients with CCM with distal recurrence and metastasis that initially recurred from 10 weeks to 17 years after initial presentation with a median time of 6 years. With this result, we cannot judge whether these patients tend to recur sooner or later than patients with CCM with only local recurrence. Moreover, 73%
of the patients (8/11) identified in the literature review presented with distal recurrence or metastasis prior to any
local recurrence. This result supports the findings of a previous study,[7] which showed that distal recurrence and metastasis does not necessarily occur only as the last step of disease progression.
The prognosis of patients with CCM is worse than those with WHO grade I and other WHO grade II meningiomas with a 5-year progression-free survival rate of 37% to 41.8%.[2,5] However, the 5-year and 10-year overall survival rates are 91% and 87%, respectively, which are similar to previous reports on atypical and choroid meningiomas. There are insufficient data on the long- term outcomes of patients with distal recurrence or metastasis in CCM. Only one 16-year-old patient reported by Zorludemir [4] died of the disease, 27 months after the initial diagnosis.
The treatment plan for patients with CCM with distal relapse or metastasis is similar to that for other patients diagnosed with CCM. The primary goal is total resection of the tumor, which is one of the most important prognostic factors in reducing recurrence.[2,5,6] Postoperative adjuvant radiotherapy is recommended, which can also lower the recurrence rate.[2,5,6] Tao et al. [5] reported
Fig. 5
A B C D
Postoperative intraspinal magnetic resonance imaging (MRI). MRI three days after spine surgery showing total removal of L3 tumor, while other remnant intraspinal tumors visible.
(A) T2 sagittal image. (B) T2 sagittal image. (C) T1-enhance sagittal image. (D) T1-enhance sagittal image.
that intraspinal CCM tends to have lower recurrence rates than intracranial lesions if totally resected. However, even after postoperative adjuvant radiotherapy, distal relapse or metastasis can occur, as in our case.
Although there are studies advocating the predictive value of risk factors associated with recurrence of CCM, as mentioned above, further validation is needed. Moreover, further studies with data from longer follow-up periods and a larger number of patients are needed.
In conclusion, CCM has an aggressive nature and frequently recurs. Total resection is very important, and adjuvant radiotherapy is recommended. Although most tumors recur at the site of the previous lesion, CCM can distantly recur or metastasize throughout the central nervous system. Therefore, the whole neuraxis must be evaluated in cases of CCM.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol 2016;131:803-20.
2. Zhang H, Ma L, Wang YB, Shu C, Kuang W, Huang YA, et al. Intracranial clear cell meningiomas: Study on clinical features and predictors of recurrence.
World Neurosurg 2017;97:693-700.e11.
3. Li J, Zhang S, Wang Q, Cheng J, Deng X, Wang Y, et al. Spinal clear cell
meningioma: Clinical study with long-term follow-up in 12 patients. World Neurosurg 2019;122:e415-26.
4. Zorludemir S, Scheithauer BW, Hirose T, Van Houten C, Miller G, Meyer FB.
Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol 1995;19:493-505.
5. Tao X, Dong J, Hou Z, Hao S, Zhang J, Wu Z, et al. Clinical features, treatment, and prognostic factors of 56 intracranial and intraspinal clear cell meningiomas.
World Neurosurg 2018;111:e880-7.
6. Li P, Yang Z, Wang Z, Zhou Q, Li S, Wang X, et al. Clinical features of clear cell meningioma: A retrospective study of 36 cases among 10,529 patients in a single institution. Acta Neurochir (Wien) 2016;158:67-76.
7. Singh R, Ryan C, Chohan MO, Tisnado J, Hadjigeorgiou GF, Bilsky MH.
Intracranial meningioma with vertebral or intraspinal metastasis: Report of 2 cases and review of the literature. J Neurosurg Spine 2016;25:775-81.
8. Carrà S, Drigo P, Gardiman M, Perilongo G, Rigobello L. Clear cell meningioma in a 22-month-old male: Update after five years. Pediatr Neurosurg 2003;38:162-3.
9. Dhall SS, Tumialán LM, Brat DJ, Barrow DL. Spinal intradural clear cell meningioma following resection of a suprasellar clear cell meningioma. Case report and recommendations for management. J Neurosurg 2005;103:559-63.
10. Imlay SP, Snider TE, Raab SS. Clear-cell meningioma: Diagnosis by fine- needle aspiration biopsy. Diagn Cytopathol 1998;18:131-6.
11. Jallo GI, Kothbauer KF, Silvera VM, Epstein FJ. Intraspinal clear cell meningioma: Diagnosis and management: Report of two cases. Neurosurgery 2001;48:218-21; discussion 221-2.
12. Nouri H, Abid L, Ouertatani M, Hentati D, Mestiri M, Jaafoura H. Delayed manifestation of sacral clear cell meningioma distent metastasis or multifocal disease? Surg Sci 2011;2:297-302.
13. Zhang J, Shrestha R, Li J, Shu J. An intracranial and intraspinal clear cell meningioma. Clin Neurol Neurosurg 2013;115:371-4.
14. Lee W, Chang KH, Choe G, Chi JG, Chung CK, Kim IH, et al. MR imaging features of clear-cell meningioma with diffuse leptomeningeal seeding. AJNR Am J Neuroradiol 2000;21:130-2.
15. Scognamiglio G, D'Antonio A, Rossi G, Cavazza A, Camerlingo R, Pirozzi G, et al. CD90 expression in atypical meningiomas and meningioma metastasis. Am J Clin Pathol 2014;141:841-9.