w w w . r e v i s t a n e f r o l o g i a . c o m RevistadelaSociedadEspañoladeNefrología
Original
article
Persistent
antiphospholipid
antibodies
are
associated
with
thrombotic
events
in
ANCA-associated
vasculitis:
A
retrospective
monocentric
study
Juyoung
Yoo
a,
Sung
Soo
Ahn
a,
Seung
Min
Jung
a,
Jason
Jungsik
Song
a,
Yong-Beom
Park
a,b,
Sang-Won
Lee
a,b,∗aDivisionofRheumatology,DepartmentofInternalMedicine,YonseiUniversityCollegeofMedicine,Seoul,RepublicofKorea bInstituteforImmunologyandImmunologicalDiseases,YonseiUniversityCollegeofMedicine,Seoul,RepublicofKorea
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received4June2018 Accepted31October2018 Availableonline21February2019
Keywords: Antineutrophilcytoplasmic antibody Vasculitis Thrombosis Antiphospholipidantibody
a
b
s
t
r
a
c
t
Aim: Weinvestigatedwhetherpersistentantiphospholipidsyndrome(APLAs)atdiagnosis areassociatedwiththeriskofthromboticeventsduringfollow-upinpatientswith ANCA-associatedvasculitis(AAV).
Methods:Weretrospectivelyreviewedthemedicalrecordsof138AAVpatients.Thrombotic eventsweredefinedasarterialandvenousthrombosisconfirmedbymagneticresonance imaging,computedtomography,angiographyandDopplerultrasonography.Clinicaland laboratoryvariablesatdiagnosisandduringfollow-upbetweenpatientswithandwithout thromboticeventswerecompared.TheunivariableandmultivariableCoxhazardmodel analysetoappropriatelyobtainhazardratio(HR)consideringthefollow-updurationwere conducted.
Results:Themeanageof138AAVpatientswas55.1yearsand44weremale.Persistent APLAsweredetectedin18patientswithAAV(13.0%).Thromboticeventswereobservedin 26patientswithAAV(18.8%)duringfollow-up.Atthetimeofaretrospectivestudypoint, persistentAPLAsatdiagnosiswereobservedmorefrequentlyinpatientswiththrombotic eventsthanthosewithout.InthemultivariableCoxhazardmodelanalysis,ageatdiagnosis (HR1.075)andpersistentAPLAs(HR2.902),butnotANCAs.Thromboticeventswere iden-tifiedmorefrequentlyinpatientswithpersistentAPLAsatdiagnosisthanthosewithout (38.9%vs.15.8%,relativerisk3.383).
Conclusion: PersistentAPLAsatdiagnosisaresignificantlyassociatedwiththeriskof throm-boticeventsduringfollow-upofAAV.Wesuggestthatphysiciansshouldcloselymonitor thedevelopmentofthromboticeventsduringfollow-up.
©2019SociedadEspa ˜noladeNefrolog´ıa.PublishedbyElsevierEspa ˜na,S.L.U.Thisisan openaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/ by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mailaddress:sangwonlee@yuhs.ac(S.-W.Lee).
https://doi.org/10.1016/j.nefro.2018.10.014
0211-6995/©2019SociedadEspa ˜noladeNefrolog´ıa.PublishedbyElsevierEspa ˜na,S.L.U.ThisisanopenaccessarticleundertheCC BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Los
anticuerpos
antifosfolípidos
persistentes
se
asocian
a
eventos
trombóticos
en
la
vasculitis
asociada
con
ANCA:
un
estudio
monocéntrico
retrospectivo
Palabrasclave: Anticuerpoanticitoplasma deneutrófilos Vasculitis Trombosis Anticuerpoantifosfolípidor
e
s
u
m
e
n
Objetivo: Seinvestigósi elsíndrome antifosfolipídico (SAF)persistente enelmomento deldiagnósticoseasociaaunriesgodeeventostrombóticosduranteelseguimientoen pacientesconvasculitisasociadaconANCA(VAA).
Métodos:Serevisarondeformaretrospectivalashistoriasclínicasde138pacientesconVAA. Loseventostrombóticossedefinieroncomoaquellosdetrombosisarterialyvenosa confir-madamedianteresonanciamagnética,tomografíacomputarizada,angiografíaoecografía Doppler.Secompararonlasvariablesclínicasyanalíticasenelmomentodeldiagnóstico y duranteelseguimiento entrelospacientescon ysineventostrombóticos.Serealizó unanálisisconunmodeloderiesgosdeCoxunivariableymultivariableparaobtenerde formaadecuadaelcocientederiesgosinstantáneos(hazardratio[HR])teniendoencuenta laduracióndelseguimiento.
Resultados:Laedadmediadelos138pacientesconVAAfuede55,1a ˜nos,y44pacientes eranvarones.SedetectóSAFpersistenteen18pacientesconVAA(13,0%).Seobservaron eventostrombóticosen26pacientesconVAA(18,8%)duranteelseguimiento.Enunpuntode análisisretrospectivodelestudioseobservóSAFpersistenteenelmomentodeldiagnóstico deformamásfrecuenteenpacientesconeventostrombóticosqueenaquellossineste tipodeeventos.EnelanálisisconunmodeloderiesgosdeCoxmultivariable,laedadenel momentodeldiagnóstico(HR:1,075)ySAFpersistente(HR:2,902),peronoANCA.Loseventos trombóticosseidentificarondeformamásfrecuenteenlospacientesconSAFpersistente enelmomentodeldiagnósticoqueenaquellossinSAF(38,9%frentea15,8%;riesgorelativo 3,383).
Conclusión:ElSAFpersistenteenelmomentodeldiagnósticoseasociódeformasignificativa aunriesgodeeventostrombóticosduranteelseguimientodelaVAA.Sesugierequelos médicosdeberíanmonitorizardeformaexhaustivaeldesarrollodeeventostrombóticos duranteelseguimiento.
©2019SociedadEspa ˜noladeNefrolog´ıa.PublicadoporElsevierEspa ˜na,S.L.U.Esteesun art´ıculoOpenAccessbajolalicenciaCCBY-NC-ND(http://creativecommons.org/licenses/ by-nc-nd/4.0/).
Introduction
Anti-neutrophilcytoplasmicantibody(ANCA)-associated vas-culitis(AAV)isa groupofsystemicvasculitides, whichare characterisedbynecrotisingvasculitiswithfeworno depo-sitionofimmune complexand byencroachingsmall sized vesselsfromcapillariestoarterioleandvenules.1ANCA con-sistsofmyeloperoxidase(MPO)-ANCAorperinuclear(P)-ANCA andproteinase3(PR3)-ANCAorcytoplasmic(C)-ANCA.2AAV canbecategorisedasthreevariantsaccordingto histologi-calfindings,ANCApositivityandclinicalfeatures:microscopic polyangiitis(MPA),granulomatosiswithpolyangiitis(GPA)and eosinophilicGPA(EGPA).1,3–5GPAoftenexhibitsitssurrogate cluesincludingupperorlowerairwaysymptomsfrom sinusi-tistocavitarynoduleinlungsandgranulomaonhistologyand EGPAismainlyassociatedwithasthma,eosinophilia, migra-torylunglesions,neuropathyandeosinophilicdegranulation orgranulomaonhistology.1,3–5Meanwhile,MPAisrelatedto renalvasculitisanddiffusealveolarhaemorrhage,butshows nogranulomaoreosinophilicinfiltrationonhistology.1,3
Antiphospholipid syndrome (APS) is characterised by vascular thrombosis ranging from veins to arteries and recurrent spontaneous abortion predominantly between 10 and 34 intrauterine weeks under persistent antiphos-pholipid antibodies (APLAs) with an interval of 12 weeks or greater.6 APLAs include anti-cardiolipin (aCL) IgM or IgG, anti-beta2glycoprotein 1(anti-2GP1)IgM and IgG and lupus anticoagulant (LAC). APS classification can be done when one clinical item or more and one laboratory items ormorearefulfilled.6,7 BecausepersistentAPLAscan accel-erate atherosclerosis and immune-mediated endothelial inflammation, they may provoke serious cerebrovascular, cardiovascular and peripheral vascular thrombosis.8 Thus, regardlessofAPSdiagnosis,persistentAPLAs mayincrease the risk of arterial and venous thrombotic events in AAV patients. A previous study reported that persistentAPLAs, particularly LAC, were positively proportional to vasculitis damagesinAAVduringfollow-up.9However,sofar,therewas noreportontheassociationbetweenpersistentAPLAsand thromboticeventsinaconsiderablenumberofpatientswith AAV.Hence,inthisstudy,weinvestigatedwhetherpersistent
APLAsatdiagnosisareassociatedwiththeriskofthrombotic eventsduringfollow-upinAAVpatients.
Objectives
Inthis study,we investigatedwhether persistentAPLAs at diagnosisare associatedwiththeriskofthromboticevents duringfollow-upinAAVpatients.
Materials
and
methods
Patients
Weretrospectively reviewedthe details of medicalrecords of 138 AAV patients according to the inclusion criteria as follows:(i) patients who were first classified as AAV from October2000toSeptember2017atDivisionofRheumatology, DepartmentofInternal Medicine,YonseiUniversity College ofMedicine,SeveranceHospital;(ii)patientswhofulfilledthe AmericanCollegeofRheumatology1990criteriaforthe classi-ficationofGPAandEGPAandthenreclassifiedbythealgorithm suggested by the European Medicines Agency in 2007, in whichauthorsaddedthemodifiedcontentsoftheChapelHill ConsensusConferences (CHCC) Nomenclature ofVasculitis proposedin20121,3–5;(iii)patientswhohadbeenfollowedup for12weeksorgreaterafterthesecondresultsofAPLAs10;(iv) patientswhohadwell-documentedmedicalrecordstoassess Birminghamvasculitis activityscore (BVAS)and prognostic fivefactorscore(FFS)(2009)atdiagnosis11–13;(v)patientswho hadtheresultsofbloodtestsforperinuclear (P)-ANCAand cytoplasmic(C)-ANCAormyeloperoxidase(MPO)-ANCAand proteinase3(PR3)-ANCAatdiagnosis,regardlessofpositivity2; (vi)patientswhohadnotbeenclassifiedasdefiniteAPSprior tothisstudy;(vii)patientswhohadnomedicalhistorytoaffect eitheritemsofBVAS,ANCApositivityorlaboratoryresults, suchasmalignancies,haematologicaldisorders,chronicliver diseases and autoimmune diseases other than AAV,which wereidentifiedinthe10threvisedInternationalClassification Diseases(ICD-10);(viii)patientswhohadneverreceiveddrugs forthosemedicalconditions(viandvii)atdiagnosis,which weresearchedbytheKoreanDrugUtilisationReview(DUR) system.ThisstudywasapprovedbytheinstitutionalReview BoardofSeveranceHospital(4-2017-0673),andthepatient’s writteninformedconsentwaswaivedbytheapprovingIRB, asthiswasaretrospectivestudy.
Clinicaldata,comorbiditiesandmedications
Wecollectedage,gender,smokinghistoryandthefollow-up duration.Thefollow-updurationwasdefinedastheperiod from diagnosis to the first thrombotic events for patients withthromboticeventsand thatfromdiagnosis tothelast visit for patients without. Weobtained clinical manifesta-tionsatdiagnosis basedonorgan-specificdetaileditemsof BVAS,11,12 and calculated it. ANCAs and APLAs at diagno-siswerecollected.Wesearchedmedicalconditionsregarding comorbiditiesduringfollow-upbyICD-10.Wealsocollected
medications administered during follow-up by under the KoreanDURsystem.
PersistentAPLAs
PersistentAPLAswasdefined,whenanytypeofAPLAs,such asaCLIgMorIgG,anti-2GP1IgMandIgGandLAC,isserially detectedwithanintervalof12weeksorgreater.
Thromboticevents
Thromboticeventsweredefinedasarterialandvenous throm-bosisconfirmedbymagneticresonance imaging,computed tomography,angiographyandDopplerultrasonography.And they werecategorised ascardiovascular or peripheral arte-rialthromboticevents,cerebrovascularthromboticeventsand venousthromboticevents.
Statisticalanalyses
AllstatisticalanalyseswereconductedusingSPSSsoftware (version23forwindows;IBMCorp.,Armonk,NY,USA). Contin-uousvariableswereexpressedasmean±standarddeviation, andcategoricalvariablesweredoneasnumberandthe per-centage. Significant differences between patients with and without thrombotic events were compared using the chi squareandFisher’s exacttestsforcategoricaldataand the Mann–Whitney U testfor continuousvariables. The multi-variable Cox hazard model using variables with statistical significancewasconductedinunivariableCoxhazardmodel to appropriatelyhazard ratio (HR).Therelativerisk (RR)of thepresenceofpersistentAPLAsforthrombosiswasanalysed usingcontingencytablesandthechisquaretest.P-valuesless than0.05wereconsideredstatisticallysignificant.
Results
Baselinecharacteristicsof138AAVpatients
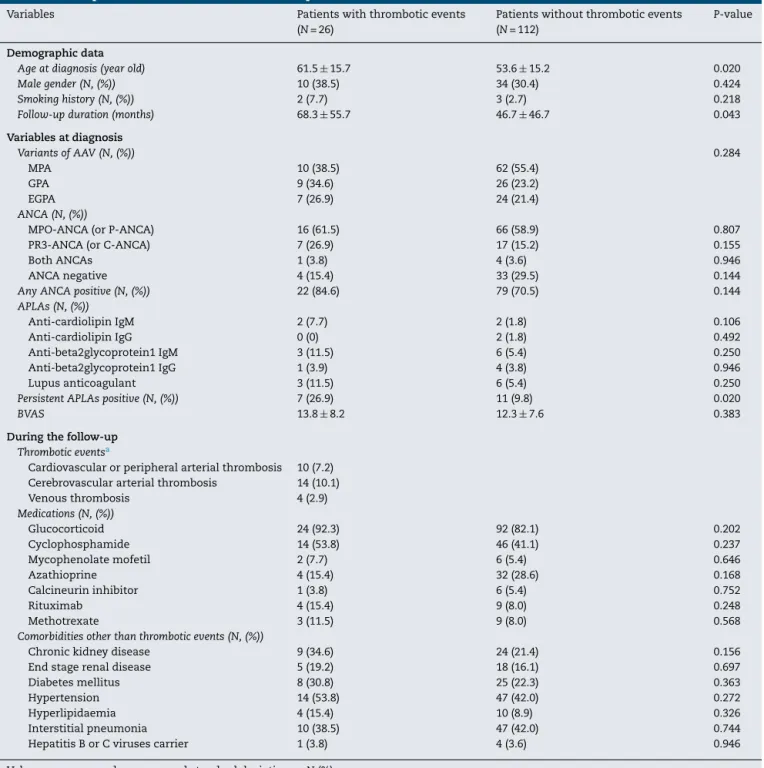
Themeanageof138AAVpatientswas55.1yearsand44were male.Themeanfollow-updurationwas50.8months. Seventy-twopatients(52.2%)hadMPA,35patients(25.4)hadGPAand 31patients(22.5%)hadEGPA.TheinitialmeanBVASandFFS (2009)were12.6and1.2.Amongcomorbidities,hypertension wasthemostfrequentlyobserved.Thromboticeventswere observedin26patientswithAAV(18.8%)duringthefollow-up (Table1).
Comparisonofvariablesatdiagnosisbetweenpatients withandwithoutthromboticevents
Whenwedivided138AAVpatientsaccordingtothrombotic events, twenty-six patients were belonging to throm-botic event group. Of26 patients with thrombotic lesions, 10patients(7.2%)exhibitedcardiovascularorperipheral arte-rialthrombosis,14patients(10.1%)exhibitedcerebrovascular thromboticlesionsand4patients(2.9%)hadvenous throm-bosis. Two patients exhibited 2 thrombotic events.Among variables at diagnosis, patients with thrombotic events
Table1–ComparisonofvariablesbetweenAAVpatientswithandwithoutthromboticevents.
Variables Patientswiththromboticevents
(N=26)
Patientswithoutthromboticevents (N=112)
P-value
Demographicdata
Ageatdiagnosis(yearold) 61.5±15.7 53.6±15.2 0.020
Malegender(N,(%)) 10(38.5) 34(30.4) 0.424
Smokinghistory(N,(%)) 2(7.7) 3(2.7) 0.218
Follow-upduration(months) 68.3±55.7 46.7±46.7 0.043
Variablesatdiagnosis VariantsofAAV(N,(%)) 0.284 MPA 10(38.5) 62(55.4) GPA 9(34.6) 26(23.2) EGPA 7(26.9) 24(21.4) ANCA(N,(%))
MPO-ANCA(orP-ANCA) 16(61.5) 66(58.9) 0.807
PR3-ANCA(orC-ANCA) 7(26.9) 17(15.2) 0.155
BothANCAs 1(3.8) 4(3.6) 0.946
ANCAnegative 4(15.4) 33(29.5) 0.144
AnyANCApositive(N,(%)) 22(84.6) 79(70.5) 0.144
APLAs(N,(%)) Anti-cardiolipinIgM 2(7.7) 2(1.8) 0.106 Anti-cardiolipinIgG 0(0) 2(1.8) 0.492 Anti-beta2glycoprotein1IgM 3(11.5) 6(5.4) 0.250 Anti-beta2glycoprotein1IgG 1(3.9) 4(3.8) 0.946 Lupusanticoagulant 3(11.5) 6(5.4) 0.250
PersistentAPLAspositive(N,(%)) 7(26.9) 11(9.8) 0.020
BVAS 13.8±8.2 12.3±7.6 0.383
Duringthefollow-up
Thromboticeventsa
Cardiovascularorperipheralarterialthrombosis 10(7.2) Cerebrovasculararterialthrombosis 14(10.1)
Venousthrombosis 4(2.9) Medications(N,(%)) Glucocorticoid 24(92.3) 92(82.1) 0.202 Cyclophosphamide 14(53.8) 46(41.1) 0.237 Mycophenolatemofetil 2(7.7) 6(5.4) 0.646 Azathioprine 4(15.4) 32(28.6) 0.168 Calcineurininhibitor 1(3.8) 6(5.4) 0.752 Rituximab 4(15.4) 9(8.0) 0.248 Methotrexate 3(11.5) 9(8.0) 0.568
Comorbiditiesotherthanthromboticevents(N,(%))
Chronickidneydisease 9(34.6) 24(21.4) 0.156
Endstagerenaldisease 5(19.2) 18(16.1) 0.697
Diabetesmellitus 8(30.8) 25(22.3) 0.363
Hypertension 14(53.8) 47(42.0) 0.272
Hyperlipidaemia 4(15.4) 10(8.9) 0.326
Interstitialpneumonia 10(38.5) 47(42.0) 0.744
HepatitisBorCvirusescarrier 1(3.8) 4(3.6) 0.946
ValuesareexpressedasmeanandstandarddeviationorN(%).
a Of26patientswiththromboticevents,twopatientsexhibitedtwothromboticevents.
AAV:antineutrophilcytoplasmicantibody(ANCA)-associatedvasculitis;MPA:microscopicpolyangiitis;GPA:granulomatosiswith polyangi-itis;EGPA:eosinophilicgranulomatosiswithpolyangiitis;MPO:myeloperoxidase;P:perinuclear;PR3:proteinase3;C:cytoplasmic;APLAs: antiphospholipidantibodies;BVAS:Birminghamvasculitisactivityscore.
exhibitedthe higher mean ageand the follow-up duration thanthosewithout(61.5vs.53.6yearsoldand68.3vs.46.7 months).TherewerenosignificantdifferencesinAPLA-types includingaCLIgMandIgG,anti-2GP1IgMandIgGandLAC. However,whenwedefinedthepresenceofanytypeofAPLAs aspersistent APLAs, persistentAPLAs were detected more frequently in patients with thrombotic events than those without (26.9% vs. 9.8%).However, any typeof ANCA was detectedevenlyinthetwogroups.Therewerenosignificant
differences in medications and comorbidities between patientswithandwithoutthromboticevents(Table1).
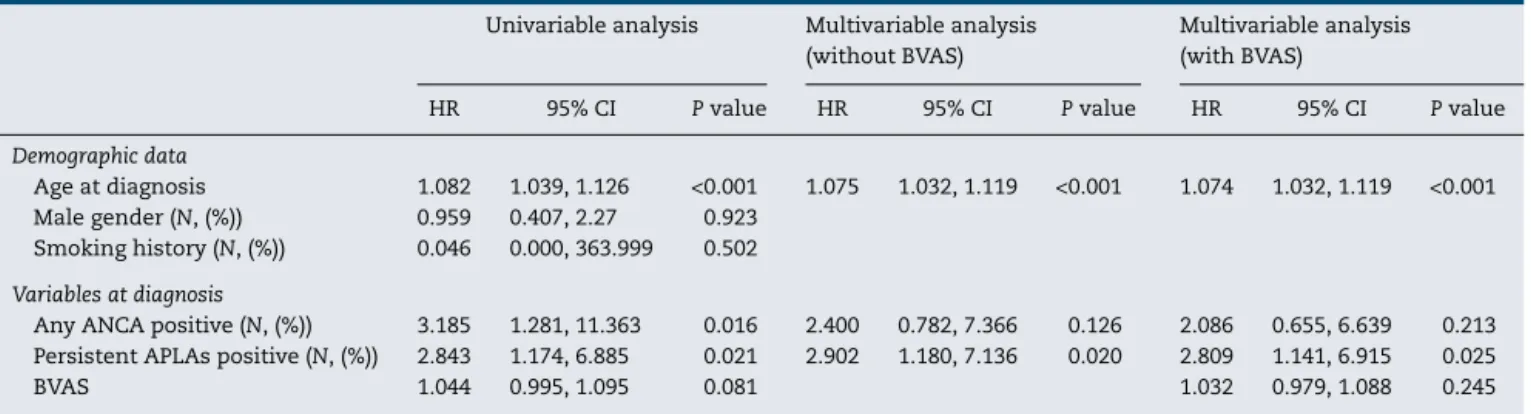
PredictivevalueofpersistentAPLAsforthromboticevents
Consideringthefollow-upduration,weperformedunivariable Coxhazardmodelanalysiswithallvariablesatdiagnosis.In the univariableCox hazard model analysis using variables atdiagnosis,ageatdiagnosis(HR1.082,P<0.001),anyANCA
Table2–MultivariableCoxhazardmodelofvariablesatdiagnosisforthromboticeventswiththosewithsignificance inunivariableanalysisinpatientswithAAV.
Univariableanalysis Multivariableanalysis (withoutBVAS)
Multivariableanalysis (withBVAS)
HR 95%CI Pvalue HR 95%CI Pvalue HR 95%CI Pvalue
Demographicdata
Ageatdiagnosis 1.082 1.039,1.126 <0.001 1.075 1.032,1.119 <0.001 1.074 1.032,1.119 <0.001
Malegender(N,(%)) 0.959 0.407,2.27 0.923
Smokinghistory(N,(%)) 0.046 0.000,363.999 0.502 Variablesatdiagnosis
AnyANCApositive(N,(%)) 3.185 1.281,11.363 0.016 2.400 0.782,7.366 0.126 2.086 0.655,6.639 0.213 PersistentAPLAspositive(N,(%)) 2.843 1.174,6.885 0.021 2.902 1.180,7.136 0.020 2.809 1.141,6.915 0.025
BVAS 1.044 0.995,1.095 0.081 1.032 0.979,1.088 0.245
AAV:antineutrophilcytoplasmicantibody(ANCA)-associatedvasculitis;BVAS:Birminghamvasculitisactivityscore;HR:hazardratio;CI: con-fidenceinterval;APLAs:antiphospholipidantibodies.
positiveatdiagnosis(HR3.185,P=0.016)andpersistentAPLAs positiveatdiagnosis(HR2.843,P=0.021).BVASatdiagnosis (HR1.044,P=0.081)tendedtobeassociatedwiththrombotic event-development, but it was not significant.In the mul-tivariableCox hazard model analysisusing three variables withsignificanceintheunivariableanalysis,ageatdiagnosis (HR 1.075, 95% confidence interval [CI] 1.032, 1.119) and persistentAPLAspositiveatdiagnosis(HR2.902,95%CI1.180, 7.136).Any ANCApositive atdiagnosis wasnot associated withthromboticeventsduringfollow-upinAAVpatients.In addition, whenweincluded BVASinthe multivariableCox hazardmodelanalysis,onlyageatdiagnosis(HR1.074,95% CI1.032,1.119)andpersistentAPLAspositiveatdiagnosis(HR 2.809,95%CI1.141,6.915)(Table2).
RelativeriskofpersistentAPLAsforthrombosis
WhenwedividedAAVpatientsintotwogroupsaccordingto thepresenceofpersistentAPLAs,18of138patients(13.0%) hadpersistentAPLAsatdiagnosis.Thromboticeventswere identifiedmorefrequentlyinpatientswithpersistentAPLAs atdiagnosis than thosewithout(38.9% vs.15.8%,P=0.020). Furthermore, patients with persistent APLAs at diagnosis exhibitedasignificantlyhighRRofthromboticevents com-paredtothosewithout(RR3.383,95%CI1.164,9.831)(Fig.1).
Discussion
Inthis study,we investigatedwhether persistentAPLAs at diagnosisare associatedwiththeriskofthromboticevents duringfollow-up.Wedemonstratedthat,atthetimeofa ret-rospective study point, persistent APLAsat diagnosis were observedmorefrequentlyinpatientswiththromboticevents thanthosewithout.Furthermore,wedrewtheconclusionthat persistentAPLAsatdiagnosis were associatedwith throm-boticeventsduringfollow-up,comparabletoageatdiagnosis. Malegender,hypertension,diabetesmellitus,dyslipidaemia andchronicrenaldiseases,whichareconventionalrisk fac-torsforcardiovascularorcerebrovasculardiseasesingeneral population,14 were notrelatedtothrombotic eventsinthis studypopulation. 100 84.2% RR 3.383 P = 0.020 61.1% 38.9 15.8% 80 60 40 20 0
(%) Persistent APLAs (−) Persistent APLAs (+) No Thrombosis Thrombosis
Fig.1–RelativeriskofpersistentAPLAsforthrombosis. Thromboticeventswereidentifiedmorefrequentlyin patientswithpersistentAPLAsatdiagnosisthanthose without.Furthermore,patientswithpersistentAPLAsat diagnosisexhibitedasignificantlyhighRRofthrombotic eventscomparedtothosewithout.APLAs:
antiphospholipidantibodies;RR:relativerisk.
PersistentAPLAscanprovokethrombogenicstateby driv-ing endothelial cells to express adhesion molecules and up-regulate tissuefactor productionalong with monocytes and enforcing platelets to produce glycoprotein 2b-3a and thromboxane A2 production.8 Persistent APLAs can also advance thrombogenic state to thrombosis state by acti-vating complement-cascades system and augmenting the interaction with coagulation-regulatory proteins.8 On the other hands, the severity ofAAV is considered associated with platelet activation markers and coagulation or fibri-nolysis indices.15,16 Thus, theoretically, all kinds of APLAs can increase the risk of thrombotic complication in AAV patients at a high rate. In this study, we elucidated that persistent APLAs atdiagnosis did affect thrombotic events during follow-up in AAV patients. Thus, we suggest that physicians should consider the need for preventive anti-aggregationoranticoagulationtherapiesinAAVpatientswith the concomitant presence ofAPLAs atdiagnosis or during follow-up.
VenousthrombosisoccurringinGPApatientsisthemost representative among vascular thrombotic events in AAV patients. A previous prospective and observational cohort studyreportedtheincidenceofvenousthrombosisas7.0per 100 person-years in180 GPA patients.17 In addition, a ret-rospectiveFrenchcohortstudyprovidedaresultsofsimilar venousthrombosisincidencesinMPAandEGPApatientsto thatinGPApatients.18Inourstudy,venousthrombosiswas observedinoneof72MPApatients(1.4%),2of35GPApatients (5.7%)and1of31EGPApatients(3.2%),buttherewasno sta-tisticalsignificance.Meanwhile,theincreasedriskofarterial thrombosisinMPA,GPAandEGPAhasbeenreported,and fur-thermorearterialthromboticeventswereoftenfoundinthe earlierphaseofAAV.19,20Inourstudy,ischaemicheartdisease wasobservedin5of72inMPApatients(6.9%),3of35GPA patients(8.6%)and2of31EGPApatients(6.5%). Cerebrovas-cularaccidentwasdone 4inMPApatients(5.6%), 5inGPA patients(14.3%)and5inEGPApatients(16.1%)without sta-tisticalsignificance.Ontheotherhands,themeanfollow-up period from diagnosis to thrombotic events in our study-patientswiththromboticeventswas68.3months,whichwas muchlongerthanthatinthosewithout.Weconcludedthat AAVtypeandtheshortfollow-up perioddidnotaffectthe incidenceofthromboticeventsinourstudy.
Ourstudyhasanadvantagethatwefirstdemonstratedthat persistentAPLAs atdiagnosis were associatedwith throm-boticeventsduringfollow-upinAAVpatients.Ourstudyalso hasseveralissues:First,becausethisstudywasdesignedasa retrospectivestudy,wecouldnotseriallymeasureAPLAs dur-ingfollow-up.Second,duetothe smallnumberofpatients withAPLAs,wecouldnotidentifytheassociationofsubtype ofAPLAswiththromboticeventsinAAVpatients.Third,given thattheprevalenceofAPLAsingeneralpopulationmight dif-feraccordingtothegeographicorethnicbackgrounds,itmust bebettertoprovidetheinformationontheminthegeneral populationinKorea,inordertoclarifythe clinical implica-tionofAPLAsinAAVpatients.However,wecouldnotcompare theprevalenceandtheclinicalimplicationofAPLAsbetween AAVpatientsandhealthypopulationduetolackofdataon thegeneralpopulationinKorea.Future prospectivestudies withlargerpatientsandserialmeasurementofAPLAstitres willprovidemoredynamicandconvincinginformationonthe influenceofpersistentAPLAsonthromboticeventsinpatients withAAV.
Conclusions
PersistentAPLAsatdiagnosisaresignificantlyassociatedwith the riskofthrombotic events duringfollow-up ofAAV. We suggest that physiciansshouldpay moreattention toAAV patients having persistent APLAs at diagnosis, encourage themtovisitthe clinicmoreoftenand closelymonitorthe developmentofthromboticeventsduringfollow-up.
Funding
ThisstudywassupportedbyafacultyresearchgrantofYonsei UniversityCollegeofMedicine(6-2016-0145).
Conflict
of
interest
Theauthorsdeclarenocompetinginterests.
r
e
f
e
r
e
n
c
e
s
1.JennetteJC,FalkRJ,BaconPA,BasuN,CidMC,FerrarioF,etal. 2012revisedinternationalChapelHillconsensusconference nomenclatureofvasculitides.ArthritisRheum.2013;65:1–11.
2.CsernokE,MoosigF.Currentandemergingtechniques forANCAdetectioninvasculitis.NatRevRheumatol. 2014;10:494–501.
3.WattsR,LaneS,HanslikT,HauserT,HellmichB,Koldingsnes W,etal.Developmentandvalidationofaconsensus methodologyfortheclassificationoftheANCA-associated vasculitidesandpolyarteritisnodosaforepidemiological studies.AnnRheumDis.2007;66:222–7.
4.LeavittRY,FauciAS,BlochDA,MichelBA,HunderGG,Arend WP,etal.TheAmericanCollegeofRheumatology1990 criteriafortheclassificationofWegener’sgranulomatosis. ArthritisRheum.1990;33:1101–7.
5.MasiAT,HunderGG,LieJT,MichelBA,BlochDA,ArendWP, etal.TheAmericanCollegeofRheumatology1990criteria fortheclassificationofChurg–Strausssyndrome(allergic granulomatosisandangiitis).ArthritisRheum.
1990;33:1094–100.
6.LevineJS,BranchDW,RauchJ.Theantiphospholipid syndrome.NEnglJMed.2002;346:752–63.
7.Gómez-PuertaJA,CerveraR.Diagnosisandclassification oftheantiphospholipidsyndrome.JAutoimmun. 2014;48–49:20–5.
8.Ruiz-IrastorzaG,CrowtherM,BranchW,KhamashtaMA. Antiphospholipidsyndrome.Lancet.2010;376:1498–509.
9.JordanN,D’cruzDP.Associationoflupusanticoagulantwith long-termdamageaccrualinantineutrophilcytoplasmic antibody-associatedvasculitis.ArthritisCareRes(Hoboken). 2016;68:711–5.
10.MukhtyarC,HellmichB,JayneD,FlossmannO,LuqmaniR. Remissioninantineutrophilcytoplasmicantibody-associated systemicvasculitis.ClinExpRheumatol.2006;24:S93–8.
11.MukhtyarC,LeeR,BrownD,CarruthersD,DasguptaB,Dubey S,etal.ModificationandvalidationoftheBirmingham VasculitisActivityScore(version3).AnnRheumDis. 2009;68:1827–32.
12.StoneJH,HoffmanGS,MerkelPA,MinYI,UhlfelderML, HellmannDB,etal.Adisease-specificactivityindexfor Wegener’sgranulomatosis:modificationoftheBirmingham VasculitisActivityScore.InternationalNetworkfortheStudy oftheSystemicVasculitides(INSSYS).ArthritisRheum. 2001;44:912–20.
13.GuillevinL,PagnouxC,SerorR,MahrA,MouthonL,Le ToumelinP,etal.,TheFive-FactorScorerevisited:assessment ofprognosesofsystemicnecrotizingvasculitidesbasedon theFrenchVasculitisStudyGroup(FVSG)cohort.Medicine (Baltimore).2011;90:19–27.
14.ExleyAR,BaconPA,LuqmaniRA,KitasGD,GordonC,Savage CO,etal.DevelopmentandinitialvalidationoftheVasculitis DamageIndexforthestandardizedclinicalassessment ofdamageinthesystemicvasculitides.ArthritisRheum. 1997;40:371–80.
15.TomassonG,LavalleyM,TanriverdiK,FinkielmanJD,DavisJC Jr,HoffmanGS,etal.Relationshipbetweenmarkersof plateletactivationandinflammationwithdiseaseactivityin Wegener’sgranulomatosis.JRheumatol.2011;38:1048–54.
16.MaTT,HuangYM,WangC,ZhaoMH,ChenM.Coagulation andfibrinolysisindexprofileinpatientswith
ANCA-associatedvasculitis.PLOSONE.2014;9:e97843.
17.MerkelPA,LoGH,HolbrookJT,TibbsAK,AllenNB,DavisJCJr, etal.Briefcommunication:highincidenceofvenous thromboticeventsamongpatientswithWegener granulomatosis:theWegener’sClinicalOccurrenceof Thrombosis(WeCLOT)Study.AnnInternMed.2005;142:620–6.
18.AllenbachY,SerorR,PagnouxC,TeixeiraL,GuilpainP, GuillevinL,etal.Highfrequencyofvenousthromboembolic eventsinChurg–Strausssyndrome,Wegener’s
granulomatosisandmicroscopicpolyangiitisbutnot
polyarteritisnodosa:asystematicretrospectivestudy on1130patients.AnnRheumDis.2009;68:564–7.
19.MorganMD,TurnbullJ,SelametU,Kaur-HayerM,Nightingale P,FerroCJ,etal.Increasedincidenceofcardiovascularevents inpatientswithantineutrophilcytoplasmic
antibody-associatedvasculitides:amatched-paircohort study.ArthritisRheum.2009;60:3493–500.
20.SuppiahR,JudgeA,BatraR,FlossmannO,HarperL,Höglund P,etal.Amodeltopredictcardiovasculareventsinpatients withnewlydiagnosedWegener’sgranulomatosisand microscopicpolyangiitis.ArthritisCareRes(Hoboken). 2011;63:588–96.