Attribution-NonCommercial-NoDerivs 2.0 KOREA You are free to :
Share — copy and redistribute the material in any medium or format Under the follwing terms :
Attribution — You must give appropriate credit, provide a link to the license, and
indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
NonCommercial — You may not use the material for commercial purposes.
NoDerivatives — If you remix, transform, or build upon the material, you may not distribute the modified material.
You do not have to comply with the license for elements of the material in the public domain or where your use is permitted by an applicable exception or limitation.
This is a human-readable summary of (and not a substitute for) the license.
Disclaimer
Bridging the lesioned spinal cord using hydrogel
By
Le Thi Anh Hong
Major in Neurosciences
Department of Biomedical Science
The Graduate School, Ajou University
Bridging the lesioned spinal cord using hydrogel
by
Le Thi Anh Hong
Α Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of The Requirements for the Degree of
Master of Biomedical Science
Supervised by
Byung Gon Kim, MD, PhD.
Major in Neurosciences
Department of Biomedical Science
The Graduate School, Ajou University
This Certifies that the dissertation
of Le Thi Anh Hong is approved.
SUPERVISORY COMMITTEE
Eun Hye Joe
Byung Gon Kim
Eunn Young Kim
The Graduate School, Ajou University
June 26
th, 2015
i
- ABSTRACT -
Bridging the lesioned spinal cord using hydrogel
Spinal cord injury (SCI) results in permanent functional deficits due to disruption of axonal connections. Attempts to repair injured spinal cord have been focused on axon regeneration in order to re-establish connections between the brain and the spinal cord below the injury level. Contusive injury, which is the most frequent type of injury occurring in human patients, leads to a formation of cystic cavities at the lesion epicenter. The cavity formation is one of major obstacles for axonal regeneration since injured axons fail to reach caudal tissue in the absence of physical and mechanical supports from extracellular matrix (ECM). Implanting artificial scaffolds has been proposed as a promising approach, but successful bridging with scaffolding biomaterials has not been convincingly demonstrated in clinically relevant contusive SCI model. Unpredictable and irregular geometry of lesion cavities formed in this model would necessitate the use of injectable hydrogel for this purpose. In the present study, I injected temperature sensitive poly(phosphazene) hydrogel, with a sol-gel transition behavior at 37°C, into the lesion epicenter in contusive rat SCI model at 1 week after injury. The hydrogel injection almost completely prevented cavity formation. In animals with the hydrogel injection, the lesion epicenter was replaced by fibronectin (FN)-enriched ECM by 4 weeks after the injection. The FN-positive ECM was surrounded by GFAP positive glial scars with an interface laden with chondroitin sulfate proteoglycans. Injection of hydrogel mixed with Taxol, which was previously reported to selectively suppress fibrotic scars, resulted in the failure of bridging cavities, suggesting a role of ECM produced by fibroblasts in the hydrogel effects. Interestingly, zymography showed upregulation of MMP-9 activity in animals with the hydrogel injection, and MMP-9 was highly expressed at the center of the FN-enriched ECM. The cellular source for the MMP-9 immunoreactivity was CD11b positive macrophages. Co-localization of FN and collagen-1α1 suggested that the majority of the newly formed ECM originates from
ii
perivascular fibroblasts.Animals with hydrogel injection showed improvement in coordinated locomotion as evidenced by BBB test and Catwalk analysis. The improvement in locomotor function was accompanied by better preservation of myelinated white matter around the lesion epicenter. Our study establishes a proof of principle that the temperature-sensitive hydrogel can be used as a cavity-bridging therapy for contusive SCI. Considering the versatility of hydrogel in a sol state incorporating various drugs and cells, this approach can also be utilized as a platform for multifaceted combinatorial therapy.
iii
TABLE OF CONTENTS
ABSTRACT ... 1
TABLE OF CONTENTS ... iii
LIST OF FIGURES ... v
ABBREVIATION ... vi
I. INTRODUCTION ... 1
A. Spinal Cord Injury ... 1
B. Cavity formation and the importance of bridging lesion after spinal cord injury. ... 2
C. Bridging cavity using biomaterials ... 3
D. Injectable hydrogel for contusive injury model ... 4
E. Aims of Study ... 5
II. MATERIALS AND METHODS ... 6
1. Animal and surgical procedures ... 6
2. Poly (phosphazene) hydrogel injection ... 6
3. Tissue processing ... 6
4. Immunohistochemistry ... 7
5. Three dimensional reconstruction of lesion cavity ... 7
6. Zymography ... 7
7. Behavioral assessment ... 8
III. RESULTS ... 10
1. Hydrogel injection prevent cavity formation after contusive spinal cord injury ... 10
2. Hydrogel injection suppress microglial activation 4 week after injection ... 14
3. Remodelling of extracellular matrix by I-5 injection ... 16
4. Matrixmetallo-proteinase-9 ( MMP-9) involved in ECM remodelling ... 20
5. Perivascular fibroblasts are a major source of the newly formed ECM at 1 and 4 week ... 23
iv
6. I-5 injection promotes functional recovery ... 25 IV. DISCUSSION ... 28 V. CONCLUSION ... 31
v
LIST OF FIGURES
Figure 1. Eriochrome and Eosin staining of spinal cord cross sections 4 weeks after injection
..………...12
Figure 2. There dimensional reconstruction of lesion cavity. ... 13
Figure 3. I-5 injection suppressed microglial activation……….…15
Figure 4. Remodelling of matrix by hydrogel injection at 1 and 4 week………18
Figure 5. FN(+) matrix is essential in the prevention of cystic cavity formation by I-5 ... 19
Figure 6. MMP-9 is highly expressed at the center of the FN (+) matrix in animals with I-5 injection………. 21
Figure 7. Perivascular fibroblasts are a major source of the newly formed ECM at 1 and 4 week………...24
Figure 8. Hydrogel I-5 injection promoted functional recovery ... 26
vi
ABBREVIATION
BBB: Blood brain barrier CD11b: intergrin, alpha M
CD68: Cluster of Differentiation 68 Col1α1: Collagen 1α1
CSPG: Chondroitin sulfate proteoglycan ECM: Extracellular matrix
FN(+): Fibronectin positive
GFAP: Glial fibrillary acidic protein HA: Hyaluronan
Iba1: Ionzied calcium binding adaptor molecule 1 MMP-2: Matrix metalloproteinase 2
MMP-9: Matrix metalloproteinase 9 PBS: Phosphate buffered saline
PDGFR-β: Platelet-derived growth factor receptor- beta SCI: Spinal cord injury
1
I.
INTRODUCTION
A. Spinal Cord Injury
Patients with spinal cord injury (SCI) suffer from life-long permanent deficits in neurological functions. Injuries occurring at high levels such as cervical injury usually lead to more severe deficits involving 4 limbs and respiration than those in lower levels of spinal cord resulting in paraplegia. Costs for care and treatment in patients with SCI is enormously huge ($3 billion each year in America) (http://www.ninds.nih.gov/disorders/sci/detail_ sci.htm). Not only does SCI cause patients themselves poor quality of life but also create economic burdens for their family and the society they belong to. The most common cause of SCI is traffic accident, besides falling, sport-related injuries, violence, and others. Among various type of injury, contusion injury is the most popular form of injury, accounting for more than 60% of SCIs (Macaya and Spector, 2012). In contusion injury, mechanical forces by compression with displaced vertebral bones are rapidly inflicted upon the spinal cord, leading to disorganization of spinal cord tissue. Although most of axons in the affected area are severed in the lesion epicenter, a certain extent of viable and intact axons are still observed at the injury site depending on the degree of injury.
Until present, there is no effective therapeutic strategy to treat SCI. Incomplete injury results in axonal disruption accompanied by damages to the remaining white matter. Attempts to repair SCI have been centered on axon regeneration to reestablish the lost connections between the brain and the nerve below the injury site. Not only do injured axons in center nervous system (CNS) have low capacity to regrowth but also hostile environment after injury inhibit their regeneration. Thus, effective treatment of SCI leading to function recovery would ultimately require multifaceted strategies. SCI triggers a series of molecular and cellular alternations resulted in cystic cavities formation (Fitch et al., 1999). In human contusive SCI, a cystic cavity was observed, and a similar pattern of cavity formation was seen in rat contusive injury while cavity does not form after mice SCI. The exact mechanism why cystic cavities develop in contusive injury still remains to be elucidated.
2
B. Cavity formation and the importance of bridging lesion after spinal cord injury.
In CNS, ECM acts as a framework that supports cell migration, synaptogenesis, and axonal guidance during development. Components of ECM change dynamically in shape and design in response to alterations of environment. ECM found in CNS is rich in proteoglycans and glycoproteins that are attached in hyaluronic acid (HA). Particularly, sulphated proteoglycans attached to tenascins and linked with HA by link proteins are densely distributed in intercellular spaces, or accumulated into condensed structures which cover presynaptic nerve terminal, surrounds perineuronal nets (PNNs) and node of Ranvier. ECM has vital role in regulating important molecular and cellular activities in physical condition (Burnside and Bradbury, 2014). However, following injury, the composition and expression of ECM proteins are changed and the alternations of ECM following injury can have unfavorable ramifications on neural repair.
Primary damages from physical injury are comprised of hemorrhage, ischemia, and unbalanced ionic homeostasis, and so forth. The primary insult is followed by secondary degenerative processes. For example, inflammatory cells infiltrate into the lesion site eliciting cellular demise via apoptosis or necrosis. Oligodendrocytes in the remote white matter are also susceptible to delayed death, contributing to demyelination and degeneration of the white matter (El Waly et al., 2014). One of the consequences of the secondary degenerative processes is a formation of fluid-filled cavity. Exact mechanisms by which cystic cavities develop following contusion injury remain to be clarified. It is hypothesized that post injury inflammatory reactions activate matrix remodeling enzymes, which in turn degrade ECM proteins leading a formation of cystic spaces (Fitch et al., 1999). Several studies have investigated mechanism of cavity formation. It was shown that inflammation alone can trigger secondary tissue damage culminating in cavitation. In addition, disruption of vascular supply may exacerbate cavitation process (Rooney et al., 2009), however the question of how this cavity develop remains largely unknown.
The expansion of cystic cavities are inevitably associated with disruption of passing axons and further loss of glial cells and spinal neurons in the parenchyma. The cavities are usually surrounded by astrocytic glial scars and chondroitin sulfate proteoglycan (CSPG) produced from them, which are known to potently impede axon regeneration (Kwok et al., 2008). More importantly, injured axons would have great difficulty in growing within fluid
3
filled cavities due to the lack of mechanical and physical supports from ECM proteins. Therefore, the formation of cystic cavities presents an onerous challenge not only for prevention of secondary damage but also for inducing regeneration of axon. There was an attempt to attenuate necrosis aiming at reducing cavity formation without a meaningful success (Fujiki et al., 2004). I speculate that developing effective strategies to prevent cavity formation or to provide bridging materials to fill the cavity spaces would be imperative for patients with spinal cord injury.
C. Bridging cavity using biomaterials
Various biomaterials have been used in past decades aiming at bridging the lesion cavity and thus at constituting extracellular matrix framework for the support of axon growth. Basically, they can be classified following their physical or chemical properties including natural or artificial scaffolds, solid or injectable, biodegradable or non-degradable materials (reference should be added). Biomaterials that are suited for being implanted into lesioned spinal cord should have certain properties for ideal outcomes. One of the important properties is biocompatibility with the host tissue. It is important not to elicit any immune reaction or produce toxic effects on host tissue or cells over a long period of time. Biomaterials also should not produce further damage when being introduced to the spinal cord. In this regard, being injectable rather solid would be advantageous since injectable hydrogel would not require surgical procedure for implantation. Last but not least, biomaterials should be degraded at the same time of tissue formation otherwise it may be toxic and become barrier for tissue regeneration. On the contrary, earlier degradation than necessary may cause tissue shrinkage and structural collapse, increase inflammation and scarring (Sung et al., 2004). Therefore, many studies have focused on using biodegradable scaffolds. (Friedman et al., 2002; Tabesh et al., 2009), chitosan (Nomura et al., 2008; Zahir et al., 2008), collagen (Paino and Bunge, 1991) and poly(L-lactide) are typical natural degradable materials. Time course of tissue formation and degradation rate has not been well characterized. At least in rodents, however, degradation rates on the order of weeks to months would be considered adequate because axonal growth and tissue regeneration are thought to occur with a time course ranging from three weeks to three months after injury (Mahoney and Anseth, 2006).
4
While natural scaffolds are expected to minimize immune rejection, artificial biomaterials have advantages of being readily modified by changing their physical and chemical properties. In addition, artificial scaffold can be synthetized in a large amount. Scaffolding biomaterials are being sought for biomedical application not only for their physical effects of filling for tissue defect but also to carry certain growth factors, therapeutic cells, drugs and so on (Woerly et al., 2001a; Woerly et al., 2001b). Previously, solid scaffold was used in hemi section spinal cord injury model (Teng et al., 2002). In this study, scaffold was fabricated before creating hemisected or transected injury model and spinal cord segment was dissected following exactly same size and morphology of scaffold. There were also a lot of studies that used scaffolds to bridge the lesion gaps in transection model. Spinal cord was completely transected and scaffolds were used to connect rostral and caudal stumps (Gao et al., 2013; Tsai et al., 2004). This kind of model has advantage of making sure that regenerating axons that found after implantation, originate from injured axons rather than spared axons. Although it was successful to implant scaffolds to injury site and functional recovery was observed in some degree but hemisection or transection injury model are not clinical relevant since majority of human injury occurs in contusion type. Unlike transection or hemisection injury, contusive injury would lead to a formation of cavities of unpredictable shape and geometry. Being injectable, therefore, would be a particularly critical quality for considering scaffolding biomaterials in contusion injuries. Moreover, implantation of solid framework into human spinal cord is unnatural and may pose unpredictable risks. Solid scaffold is disadvantageous also because it is hard to handle and to load peptides or drugs Furthermore, solid nature may not create good distribution network that necessary for cells, peptides, drugs, trophic factors infiltration within it. Injectable hydrogel would be an advanced material to meet these challenges.
D. Injectable hydrogel for contusive injury model
A various number of injectable materials have been produced for a variety of tissue specific applications not only for brain and spinal cord but also bone, cartilage, intervertebral disk (Kretlow et al., 2007). Hydrogels are cross-linked polymers with hydrophilic properties. They have tissue like water content, porous structure pore size 10-100µm (Pradny et al., 2006; Prang et al., 2006). Theoretically, hydrogel is in a liquid state therefore no matter how the shape of cavity is, gel can fill cavities making use of its fluidity. Liquid state of gel
5
facilitates incorporating other agents or cell. Injection of injectable hydrogels in contusion SCI would not necessitate further damage since intact meninges are not disrupted by injection. Gelation phase in vivo can provide mechanical strength mimicking native tissue.
Only several studies have used injectable hydrogel for contusion injury model. One group demonstrated that injectable hydrogel (Hyaluronan and methylcellulose) could be employed in contusive injury model (Kang et al., 2013). Despite its positive effects on preservation of blood vessel density, it failed to completely bridge the cystic cavities developed after contusion injury (Austin et al., 2012; Mothe et al., 2013). To my knowledge, there is no study that reported successful bridging of cystic cavities in contusion injury model.
Among injectable hydrogels, temperature sensitive hydrogel is a advanced material since it has rapid sol-gel transition behavior and gelation occurs in situ at 37°C (Jeong et al., 2002; Klouda and Mikos, 2008). Because the temperature-sensitive hydrogel can be maintained in a liquid state at low temperature, it is readily mixed with various soluble agents such as drugs, cells, trophic factors, enzyme, peptides, etc. Finally, gelation process would entail building up a mechanical framework that supports axon growth as well as creating a vital matrix that mimics ECM.
E. Aims of Study
Considering most suitable scaffold among various biomaterials with different advantages and disadvantages, characteristics of temperature sensitive injectable hydrogel seemed to be most optimal for the purpose of a bridging strategy in contusive spinal cord injury model. In my study, I examined potential beneficial effects of a temperature sensitive hydrogel, poly(phosphazene) hydrogel (I-5), in contusive spinal cord injury model and examined whether this bridging effect can bring out functional recovery.
6
II.
MATERIALS AND METHODS
1. Animal and surgical procedures
Aldult female SpagueDawley rats (250-300g).All animal protocols were approved by the Institutional Animal Care and Use Committee of Ajou University School of Medicine. Rats were injected with 4% chloral hydrate to be anesthetized (10 ml/kg, injected intraperitoneally), followed by a dorsal laminectomy at the 10th thoracic vertebral level (T10-11) to expose the dorsal surface of the spinal cord. The IH Impactor was used to produce standardized contusion of 200 kdyn, muscles tissues were sutured in layer, and the skin was stapled.
2. Poly (phosphazene) hydrogel injection
For hydrogel injection, animals were randomly divided into 2 groups PBS (n=6) or poly(phosphazene) hydrogel (I-5) (n=6) was provided by Korean Institute of Science and Technology, 1 week after contusion spinal cord injury, rats were re-anesthetized and the lesion sites were exposed again, 8µl of PBS or hydrogel was injected manually using manual microliter Halmington syringe (model 701RN, USA). Before injection, gel was stored on ice to prevent gelation. After injection, needle was incubated in spinal cord lesion site for 30s to prevent overflow.
3. Tissue processing
For histological assessment of lesion site, animals were killed at 1 or 4 weeks after hydrogel or PBS injection (2 or 5 weeks after injury) for early and late time points. Spinal cord tissue was taken as previously described (Hwang et al., 2014). Animals were anesthetized with 4% chloral hydrate and perfused intracardially with PBS, followed by 4.0% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Then, spinal cord was dissected and tissue block containing the epicenter ± 1 cm-long spinal cord was postfixed in 4.0 % paraformaldehyde for up to 6-8 hours, and then tissue was cryoprotected in a graded series of sucrose solutions. Sections of the spinal cord were cut transversely with 20 μm thickness using a cryostat (CM 1900; Leica) and thaw-mounted onto Super Frost Plus slides (Fisher Scientific). Slides contain spinal cord samples were dry out at 35°C for 1 hour and stored at -20°C for further examination.
7
4. Immunohistochemistry
For immunohistochemistry, spinal cord tissue sections were incubated overnight at 4°C with the following primary antibodies: anti-GFAP (1:500, chicken polyclonal, abcam), anti-fibronectin (1:100, rabbit polyclonal; Sigma), CS-56 (1:200, mouse monoclonal; Sigma), Iba-1 (1:500, rabbit polyclonal; Wako), anti-PDGFR-β (1:300, rabbit monoclonal; abcam). anti-collagen1α1 (1:100, mouse polyclonal; Santacruz), anti-MMP-9, (1:100, rabbit polyclonal; Millipore), anti-CD45 (1:500, mouse; serotec), and anti-CD11b (1:500, mouse; Serotec) antibodies. After washing three times, slides were incubated with appropriate secondary antibodies conjugated to the Alexa Fluor fluorescent dyes. Images were taken using the Olympus confocal laser scanning microscope (model FV 300).
5. Three dimensional reconstruction of lesion cavity
For quantitative analysis of cavity volume, serial spinal cord sections stained with eriochrome and eosin were three-dimensionally reconstructed. For eriochrome and eosin staining, the cross sections of spinal cord were immersed for 8 min in a staining solution consisting of 240 ml of 0.2% eriochrome cyanine RC (Sigma) and 10 ml of 10% FeCl3 · 6H2O (Sigma) in 3% HCl. The sections were then washed with running tap water, followed by differentiation in 1% NH4OH. After eriochrome cyanine staining, the sections were counterstained with eosin solution to visualize the entire spinal cord regions and lesion cavities clearly. Three dimensional reconstruction of lesion cavity was done using the Neurolucida Virtual Tissue 3D ver 10(MBF bioscience). Contours of the spinal cord outer boundary, white matter region, cystic cavity, and lesioned spinal cord tissue were manually drawn on each section, and the software generated 3D images. Different colors was assigned to distinguish white matter (white), grey matter (green), lesion (yellow), and cystic cavity (red). The volume of cystic cavities and residual tissue was was calculated automatically by the Neurolucida software.
6. Zymography
For the detection of activity of matrix metalloproteinase 2 and 9 (MMP-2 and MMM-9), PBS (N=5) and I-5 (N=4) injected animals were killed at 1 week after injection., 1 cm-long spinal cord segment with epicenter region at the center was freshly dissected and were quickly frozen at -80°C. The spinal cord tissue was homogenized and sonicated in
8
RIPA buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, and 1% SDS. Fifty µg protein from each sample was loaded onto a polyacrylamide gel containing SDS (to linearize the proteins) and gelatin (substrate for MMP-2 and MMP-9) and subjected to electrophoresis. Then, the gel was re-natured to allow proteins to regain their tertiary structure that is necessary for enzymatic activity by providing renaturing buffer (2.5% Triton X-100 and then incubated in substrate buffer (50 mM Tris-HCl, pH 8.5, 5 mM CaCl2) in 1hour followed by 3 times washing with developing buffer, 10 minute each time. Gels were transferred to fresh developing buffer (Tris base 50mM, NaCl 200mM, ZnCl2, CaCl2.2H20 5mM and NaN3 0.02%) and incubate in 37°C for 72 hours to allow the protease to digest its substrate. Then, the gel was stained by Comassi blue in 2 hours and then was destained in methanol and formic acid. After washing the excess dye off the gel, areas of protease digestion appear as clear bands. Band intensity between PBS and -I-5 group was determined by densitometry, using ImageJ software.
7. Behavioral assessment
For comparison of behavioral recovery, animals were randomly allocated to either PBS (N = 9) or hydrogel (N = 8) injection group after contusion injury. Locomotor recovery was evaluated using the Basso, Beattie and Bresnahan (BBB) open field locomotor scale and Catwalk footprint analysis. For BBB test, rats were allowed to walk freely in an open field and the BBB locomotor rating scale was determined after the 3-minute observation. Recovery of hindlimb movements was assessed 1 day after injury and then once a week for the duration of 8 weeks.
For the computerized footprint analysis, Catwalk system (Noldus Information Technology) was used. Animals were pretrained to walk on the Catwalk runway in an uninterrupted manner. On the test day, 4 runs with significant interruption were obtained. Individual footprints were determined manually using the Catwalk software (ver 7.1). Then, the software automatically calculated the following gait parameters: stride length, base of support, relative position and rotation angle. The angle of hindpaw rotation was defined as the angle (in degrees) of the hindpaw axis relative to the horizontal plane. Base of support was measured by the width between the left and right hindpaws. Left and right hindpaws were averaged to calculate the stride length and paw angle values. Footprints of hindpaws tend to overlap those of forepaws during walking in uninjured animals. However, injured
9
animals often lose this coordination between hind- and forepaws (Hamers et al., 2006). Therefore, relative position of fore- and hindpaws was obtained by directly measuring the distance between the center pads of fore- and hindpaws. Statistical analysis
Statistical analysis was performed with GraphPad Prism ver 5. Unpaired Student’s t test or one-way ANOVA was used for comparison of group means. Repeated-measures two-way ANOVAs were used to compare differences in BBB scores matched at different time points. Error bars in all graphs represent standard error of mean (SEM).
10
III.
RESULTS
1. Hydrogel injection prevent cavity formation after contusive spinal cord injury
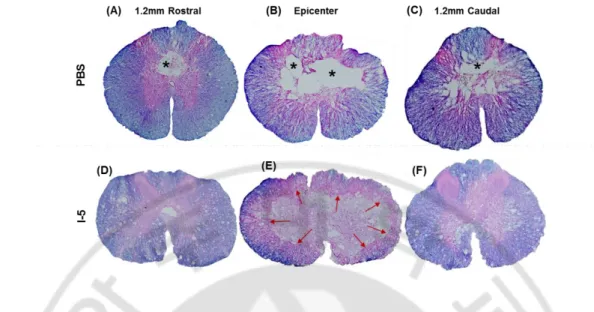
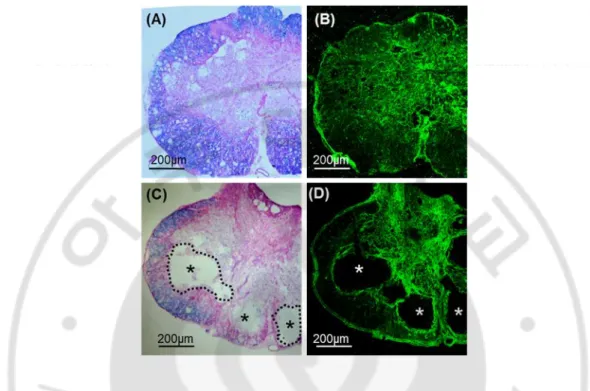
Previous studies showed that discernable cystic cavities were formed as early as 1 week after spinal cord contusion injury. Cystic cavities were progressively enlarged by 4 week time point and there was no obvious difference between the 4 and 10-week time points (Ek et al., 2010). Based on this previous report, I chose to examine cavity formation at 4-week time points after injection of PBS or Hydrogel (I-5). Injection was performed 1 4-week after contusion injury, allowing cystic space to be formed to avoid potential tissue damages related to the injection itself. Cross sections of spinal cord tissues were stained with eriochrome and eosin dyes to distinguish myelinated areas (eriochrome, blue) and extracellular matrix (ECM) (eosin, pink) from the cystic cavities (no colors). As expected, a large cystic cavity was observed at the epicenter region of animals injected with PBS (Fig. 1B), extending rostrocaudally up to 1.2 mm away from the epicenter (Fig. 1A and Fig. 1C respectively). In contrast, virtually no cavity was found at the epicenter region of animals that received I-5 injection (Fig. 1D-F). Instead of cystic cavities, eosin-stained ECM-like tissue was observed at the central region.
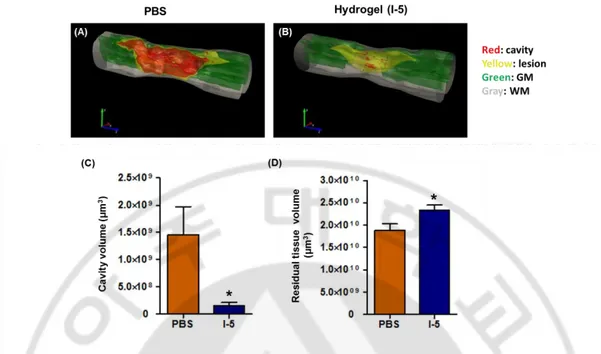
To quantitatively compare cavity volumes between the two groups, cross-sectional images of the spinal cord were reconstructed to produce three-dimensional image using the Neurolucida software. I assigned different colors to different structures; red color for lesion cavity, yellow for remaining, but damaged tissue, green for remaining and intact gray matter, and gray for remaining white matter. As can be seen in the figure, cavity volume was sharply declined in animals with I-5 injection (Fig. 2B) compared to the large volume marked as cavity in PBS injected animals (Fig. 2A). Quantification data confirmed that the cavity volume in PBS group (mean = 1.460e+009 ± 5.150e+008 N=6) was significantly decreased compared to that of I-5 group (mean = 1.507e+008 ± 5.857e+007 N=6), resulting in the reduction of cavity volume to only 10% of control value by hydrogel injection (Fig. 2C). The volume of residual tissue was calculated by subtracting the cavity volume from the volume of entire, virtual spinal cord tissue limited by outer boundaries, PBS group (mean = 1.888e+010 ± 1.384e+009 N=6 ), I-5 group (mean = 2.333e+010 ± 1.164e+009 N=6). Quantification graph showed that spare tissue volume in hydrogel injected animals (mean =
11
2.84x1010 µm3) was also significantly greater than those in PBS group (Fig. 2D). In summary, I-5 injection virtually prevented formation of cystic cavities and contributed to the preservation of spinal cord tissue after contusion injury.
12
Figure 1. Eriochrome and Eosin staining of spinal cord cross sections 4 weeks after injection. (A-C) Representative images of transverse section of spinal cord tissue obtained from animals with PBS injection. (A) 1.2mm rostral to epicenter, (B) epicenter, (C) 1.2mm caudal to epicenter. (D-F) Representative images of transverse section of spinal cord tissue obtained from animals with hydrogel injection. (D) 1.2mm rostral to epicenter, (E) epicenter, (F) 1.2mm caudal to epicenter. Spinal cord sections were stained with eriochrome and eosin dyes. * injury cavity, red arrows: eosin-stained new matrix replacing cavity space.
13
Figure 2. There dimensional reconstruction of lesion cavity. (A) 3D Image of PBS
injected animal. (B) 3D image of hydrogel injected animal. Red: cavity, Yellow: lesion, Green: grey matter, Gray: white matter. (C) Quantification of cavity volume. Orange bar indicates PBS injected group (n=6), navy bar indicates I-5 injected group (n=6). Cavity volume was significantly reduced by one-tenth by hydrogel injection. (D) Quantification graph of residual tissue volume. In I-5 group, the volume of residual tissue was significantly higher that that of PBS group. (*) represent p < 0.05 by unpaired t test.
14
2. Hydrogel injection suppress microglial activation 4 week after injection
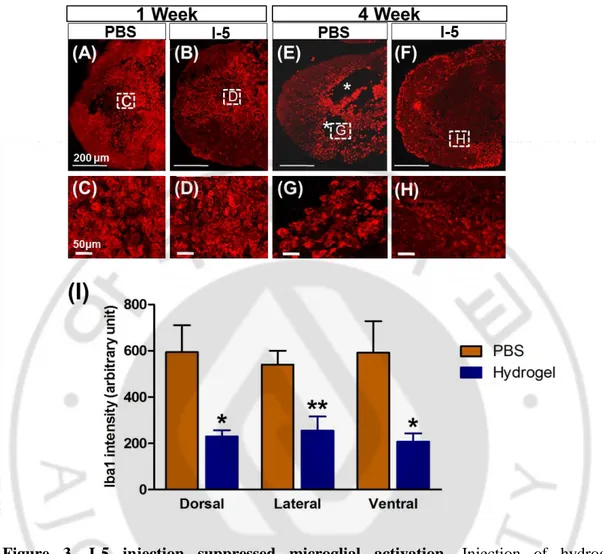
Hydrogel is artificially generated foreign material. Upon introduction to spinal cord tissue, therefore, it is likely to evoke foreign body reactions comprising activation of inflammatory cells (Kang et al., 2010). To examine the possibility of exaggerated inflammtory activation, spinal cord sections obtained at 1 or 4 weeks after injection were immunostained with microglial marker, Iba1. At 1 week after injection, intense Iba1 positive signals were observed in and around the epicenter, to a similar extent in both groups (Fig. 3A and 3B). At 4 weeks after injection, Iba-1 positive signals seemed to be concentrated around lesion cavities with signal strength not apparently attenated compared to that at 1-week time point (Fig. 3E). In animals with hydrogel injection, Iba-1 postive signals were very weak in and around the areas with new matrix replacing cavity space onspicuous cavity space (Fig. 3F). Enlarged images of Iba1 showed that morphology and intensity of Iba1 possitive macrophages was similar between PBS and I-5 group at 1 week time point (Fig. 3C and 3D), respectively. Howerver, at 4 week time point, intensity of Iba1 macrophage in I-5 group was subsided dramatically (Fig. 3H) in comparison with that in PBS group. (n=6 each group). And then intensity was evaluated by Image J. The quantification graph from the data at 4-week time point showed that the intensity of Iba-1 positive signals were significantly decreased in hydrogel group (Fig. 3I). These data suggest that injected hydrogel did not provoke foreign body inflammatory reactions, rather the hydrogel suppressed injury-related activation of microglial cells.
15
Figure 3. I-5 injection suppressed microglial activation. Injection of hydrogel
significantly reduced microglial activation at 4 week after injection. Representative images of Iba1 possitive macrophages stained at 1 week (A-D) and 4 week (E-H) after injection of PBS (A, C, E, G) or hydrogel (B, D, F, H). (C, D, G, H) higher magnification images of boxed regions in (A, B, E, F), respectively. (I) Quantification graph of Iba1 intensity (arbitrary unit). PBS or I-5 group (N = 6 for each group). (*) and (**) represent p < 0.05 and p < 0.01, respectively, by one way ANOVA followed by Tukey’s posthoc analysis. (A, B, E, and F) Scale bars represent 200 µm, (C, D, G, and H) Scale bars represent 50 µm.
16
3. Remodelling of extracellular matrix by I-5 injection
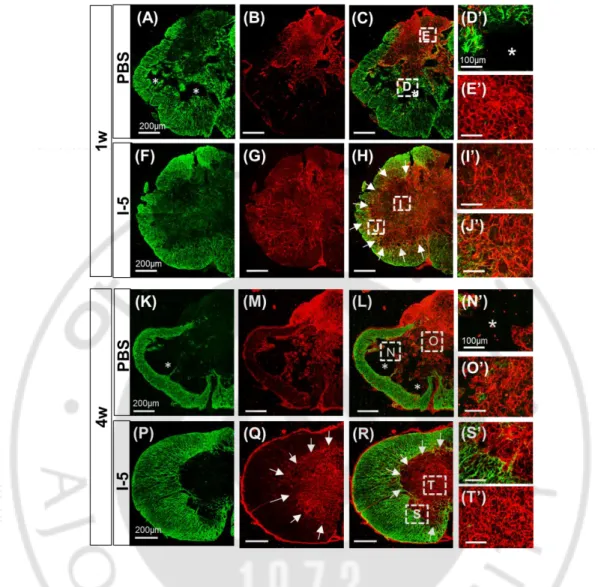
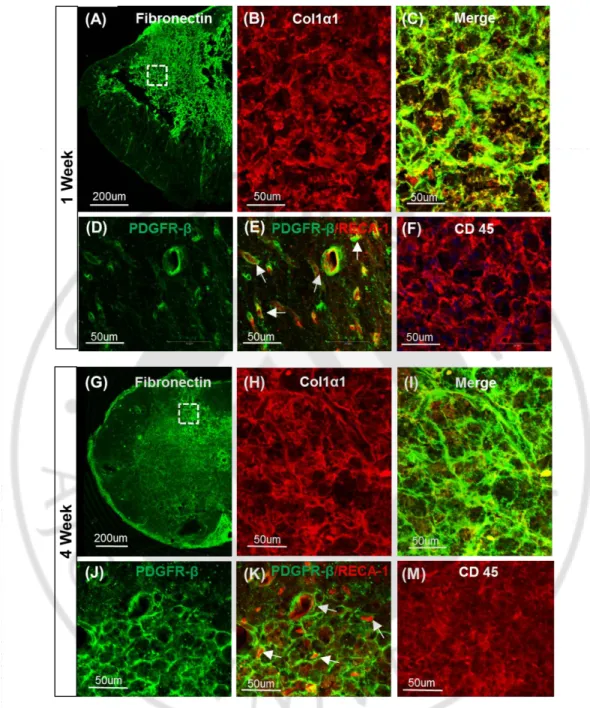
Next, I sought to characterize the ECM deposited at the epicenter region where cystic cavity is supposed to be formed without I-5 injection. At 1 week after injection, GFAP immunostaining showed increased astroglial activity at the central area of the epicenter region and formation of cystic cavity seemed to begin at this time point in animals with PBS injection (Fig. 4A, D). Fibronectin (FN) immunostaining was performed simultaneously to examine deposition of fibroblast-derived matrix. FN-positive matrix was densly observed at the lesion center (Fig. 4B,E), but the FN-positive areas tended to be segregated from GFAP-positive regions (Fig. 4C). In animals with I-5 injection, denser and more extensive FN-positive matrix occupied the lesion center (Fig. 4G,I) and GFAP-FN-positive astroglial acitvity seemed to be more clearly segregated compared to animals with PBS injection (Fig. 4F,H,J). Again, there was no evidence of cavity formation in this group. (Fig. 4F-J). At 4 weeks after injection, the majority of FN-positive matrix was replaced by cystic cavities formed at the lesion center in animals with PBS injection (Fig. 4M,N). GFAP-positive astrocytes were hardly observed in the FN-positive regions at this time point (Fig. 4K,L,O). In animals with I-5 injection, the FN-positive matrix seemed to be more concentrated and virtually occupied the entire central regions of the epicenter (Fig. 4Q,T). Segregation between GFAP-positive and FN-positive areas became solidifed at this time point, and there was a discrete boundary (white arrows in Fig. 4Q,R) between the matrix formed by GFAP-positive astrocytes and fibroblasts (Fig. 4P,R,S).
The above findings indicated that I-5 injection resulted in deposition of newly formed matrix derived from fibroblasts at the lesion epicenter, preventing formation of cytic cavities. This suggests that fibroblasts and fibroblastic scars may play a role in the I-5-induced deposition of matrix and abrogation of cavity formation. A previous study has demonstrated that microtubule stabilizer Taxol can reduce fibrotic scar. To examine if activity of fibroblast scars contributes to the 5 effects in bridging cystic cavities, I mixed I-5 with Taxol (I-5µg) and injected the mixture into the injury site. Injection of I-I-5 mixed with Taxol resulted in the failure of the deposition of FN(+) matrix and the development of cystic cavities in the leion center (Fig. 5C,D), while this phenomenon did not occur in animals with I-5 injection only (Fig. 5A,B). This result suggested that deposition of FN-positive matrix
17
derived from fibroblasts is essential in the prevention of cystic cavity formation by I-5 injection.
18
Figure 4. Remodelling of matrix by I-5 injection at 1 and 4 week. (A-J’) Representative
images at 1 week after injection. (A-E’) Fibronectin possitive matrix (red) was partly replaced by cavity spaces (*). (A) GFAP possitive astrocyte (green) confine the lesion site. (B) lesion core was occupied by FN(+) matrix with early cavitation. (C) merged image. (D’) high magnification of (D) from (C) represent cavity. (E’) magnified image of (E) from (C) indicates FN(+) matrix. (K-O’) 4 week after PBS injection, most of FN (+) matrix was replaced by cavity spaces. (K) GFAP, (M) fibronectin, (L) Merged image. (N’) magnified image of (N) from (L) illustrate cavity. (O’) high magnification of (O) from (L) represent FN(+) matrix. Scale bars from low magnification in (A) (B) (C) (F) (G) (H) (K) (M) (L) (P) (Q) (R) represent 200µm. Scale bars in (D’) (E’) (I’) (J’) (N’) (O’) (S’) (T’) represent 100µm. (A-C), (K-L).
19
Figure 5. FN(+) matrix is essential in the prevention of cystic cavity formation by I-5.
(A) (C) Representative image from I-5 injected spinal cord sections with or without Taxol administration. (A) Eriochrome and Eosin staining showed very little cavities. Myelin stained with Eriochrome (blue), Eosin stained ECM (pink). (B) (D) Sections stained with Fibronectin. Injection of I-5 mixed with Taxol failed to form FN(+) matrix. (*) indicates cavities, dotted circles represent boundary between cavity and residual tissue. All scale bars represent 200µm.
20
4. Matrixmetallo-proteinase-9 ( MMP-9) involved in ECM remodelling
The above results suggested that hydrogel injection may induce remodeling of ECM leading to prevention of cavitation and deposition of fibroblast-derived matrix. I hypothesized that ECM remodeling enzyme matrixmetalloproteinase (MMP) is invovled in this process. MMPs are zinc-endopeptidase with multiple functions involved in both pathological and physical conditions in CNS. Of all the MMP family members, MMP-9 and MMP-2 belong to gelatinase familly (Zhang et al., 2011). There is increasing evidence that gelatinase activity has benifical role in matrix remodelling and wound healing (Hsu et al., 2006; Kyriakides et al., 2009). I performed gelatinase zymography to detect gelatinase activity using spinal cord lysates obtained 1 week after injection of PBS or I-5. Compared to animals with PBS injection, MMP-9 activity was clearly enhanced in animals with hydrogel injection. Quantification graph showed that mean intensity of MMP-9 band was 7.1 ± 0.4732 N=5 in PBS groups and 16.15 ± 1.531 N=4 in I-5 group, which was 2-fold increase compared to PBS group (Fig. 6L). Quantification graph demonstrated that MMP-9 activity in I-5 group significantly surged.
I also performed immunohistochemistry to examine cellular sources for MMP-9 acitvity. As expected, MMP-9 immunoreactivity was elevated within FN-positive matrix in animals with I-5 injection (Fig. 6D-F). In contrast, there was no discernable MMP-9 immunoreactivity in remaining FN-positive matrix in animals with PBS injection (Fig. 6A-C). The majority of MMP-9 immunoreactivity was colocalized with CD11b immunoreactivity, indicating that MMP-9 was
predominantly expression in macrophage or microglial cells within FN-positive matrix.
22
Figure 6. MMP-9 is highly expressed at the center of the FN (+) matrix in animals with I-5 injection. (A) Gelatinase activity of MMP-9 and MMP-2 in PBS group (n=5) and I-5
group (n=4) examined by Zymography. MMP-9 activity was unregulated 1 week after I-5 injection compared with PBS injection. Red box indicated clear bands from I-5 group only. (B) Quantification graph showed that MMP-2 activity is not significant difference between 2 group. (C) MMP-9 activity significantly increased after I-5 injection compared with that in PBS injected animals (D-F’) Sections from animals 4w after PBS injection. MMP-9 was not observed in PBS injected animals. (D) Cavities replace FN(+) matrix (Green). (E) MMP-9 (Green) harly observed in PBS injected animals. (F’) magnified image from (F) in figure (E). (G-I’) MMP-9 expression was exclusively observed within the FN(+) matrix 4 week after injection . (G) FN(+) matrix occupied the epicenter, white arrows indicate matrix boundary. (H) MMP-9 immunoreactivity. (I’) high magnified image from (I). (J) MMP-9. (K) CD11b positive macrophages. (M) MMP-9 co-localize with CD11b suggest that hematogenous macrophages are the cellular source of MMP-9. Scale bars in (A B D E) represent 200µm. Scale bars in (E’ F’ G H I) represent 50µm. (***) represent p < 0.001, by unpaired t test
23
5. Perivascular fibroblasts are a major source of the newly formed ECM at 1 and 4 week
Previous studies showed that fibrotic scars originate from collagen 1α1 expressing perivascular fibroblasts after contusive spinal cord injury (Soderblom et al., 2013; Zhu et al., 2015b). I examined whether FN-positive matrix generated by I-5 injection also derived from the perivascular fibroblasts. Double immunostaining of fibronectin and collagen 1α1 was performed at 1 week and 4 weeks after I-5 injection. Immunoreactivity against collagen 1α1 was almost completely overlapped with FN immunoreactivity at both time points (Fig. 7A-C, G-I). Co-localization of these markers indicated that fibronectin in the new ECM may dervied from perivascular fibroblasts. PDGFR-beta is another marker for the perivascular fibroblasts. I found that PDGFR-beta immunoreactivity, which frequently encircled RECA-1 immunoreactive endothelial cells, within the FN-positive matrix at 1 week. At 4-week time point, PDGFR-beta immunoreactivity increased within the matrix, indicating that perivascular fibroblasts proliferated between the two time points (Fig. 7D,E,J,K). I also observed subtantial expression of CD45, which is marker for hematogenous macrophages, at 1-week time points (Fig. 7F). At 4 weeks after injection, intensity of CD45 was decreased compared to that observed at 1-week time point (Fig. 7M). Together, these results suggest that fibroblasts responsible for production of new ECM after I-5 injection are of perivascular origin, and abundance of CD45+ macrophages within the FN-positive matrix suggest that blood-born imacrophages play a certain role in the hydrogel-induced formation of new fibrotic matrix.
24
Figure 7. Perivascular fibroblasts are a major source of the newly formed ECM at 1 and 4 week. (A-F) 1 week after I-5 injection. (A) Fibronection immunostained spinal cord
(green). (B) Collagen 1alpha1 (red). (C) Merged image. (D) Perivascullar fibroblasts marker PDGFR-β. (E) PDGFR-β expressed around blood vessel maker RECA-1. (F) Blood borned macrophage marker CD45. (G-M) 4 week after I-5 injection.
25
6. I-5 injection promotes functional recovery
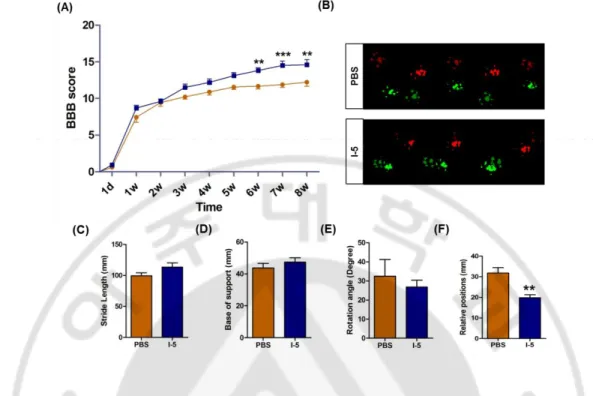
I found that I-5 injection into lesioned spinal cord can successfully bridge lesion cavity. I sought to investigate whether I-5 injection can also lead to behavioral recovery. BBB locomotor score were assessed over 8 weeks after injury (7 week after PBS or I-5 injection). Animals in both groups showed spontaneous recovery over the time. However, significant better recovery was observed in I-5 injection group at 6, 7, and 8 weeks after injury compared to animals with PBS injection. Repeated measures two-way ANOVA revealed significant influence of I-5 treatment on behavioral recovery (p <0.05), and the interaction between treatment and time points was also significant (p< 0.001). Posthoc Bonferroni test showed a significant difference between the two groups from 6- to 8-week time points (p < 0.05) (Fig. 8A). Catwalk system was used to assess quality of footprints. In I-5 group stride length tend to recover but this recovery was not significant different compared with PBS group (Fig. 8C). Following injury, the angle of hindpaw rotation relative to the body axis tends to increase indicating external rotation of hindpaws. I-5 injection did not affect the rotation angle. outward steeping. Base of support tends to widen after injury, but I-5 injection also did not affect these parameter (Fig. 8D, 8E). Co-ordinated engagement of fore- and hindpaws during locomotion was impaired after injury, resulting in non-overlapping footprints between fore- and hindpaws. This impairment can be measured by increased distance between the two footprints (defined as relative position). Taken together, these results demonstrated that injection of I-5 significantly enhanced functional recovery.
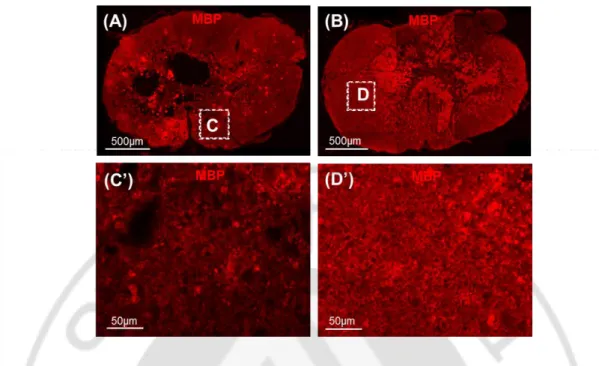
To investigate potential mechanism by which I-5 injection enhances functional outcomes, I examined myelin intensity in animals that underwent behavior tests. As expected, myelin was protected in only I-5 injection (Fig. 9A), no cavity was found in this group. In contrast, animals with PBS injection displayed lower intensity of MBP myelin staining, cavities also seen in this group. These data suggest that I-5 injection preserved myelin in the remaining white matter and that enhanced myelin preservation may underlie behavioral improvement in animals with I-5 injection.
26
Figure 8. I-5 injection promoted functional recovery. (A) Loco motor function recovery
over 7 weeks after injection. I-5 injected animals (blue) showed significant higher loco motor score than those in PBS injected animals (orange). **p<0.01; *** p<0.001 by repeated- measures two-way ANOVA followed by Tukey’s posthoc analysis at each time point. (B) Representative footprints analyzed by the Catwalk system. Colors of footprints were assigned by the Catwalk program. (bright red= left forepaw, dim red = left hindpaw, bright green = right forepaw and dim green = right hindpaw). (C-F) Quality of locomotion were evaluated by 4 parameters at 7 week after SCI. (C) stride length (distance between the two consecutive hindpaw footprints). (D) base of support (width between the left and right hindpaws. (E) Relative positions (distance between the center pads of forepaw and hindpaw prints), and (angle of the hindpaw axis relative to the horizontal plane; F). **p<0.01 by unpaired t test. PBS (n=9); I-5 (n=8).
27
Figure 9. Myelin preservation by I-5 injection correlated with functional recovery. (A,B)
MBP (Myelin basic protein) immunostaining of spinal cord cross section in animal with PBS and I-5 injection (A, B respectively). (C’) High magnification showed in the box C from (A). (D’) Magnified image taken from box (D) in (B). Scale bars of (A,B) present 500µm, (C,D) present 50µm.
28
IV. DISCUSSION
Bridging lesion cavity in SCI is one of the most important steps in injury repair. In this study I revealed that thermal sensitive hydrogel I-5 injection successfully bridged the lesion cavities. The cavity was replaced by remodeled FN(+) matrix. This matrix would reflect natural nature of tissue since I-5 is presumably degraded. FN is an important connective tissue protein, that is the reason why bioengineered biomaterials incorporated with fibronectin to guide and support nerve growth (Ahmed et al., 2003; King et al., 2006). I found that this FN-rich matrix originated from perivascular fibroblasts. The nature of this matrix is similar to that found in the lesion site in mice model where cystic cavities usually do not develop (Soderblom et al., 2013). There was a evidence that FN(+) matrix may present fibrotic scar that inhibit axon growth (Zhu et al., 2015a) and decrease of this matrix by depletion of CD11b hematogenous macrophage with clodronate liposomes resulted in an increase of neurofilament axons growth in the epicenter. In my study, reducing FN(+) matrix by I-5 plus Taxol administration led to a failure of bridging (Fig 5C,D). These data indicated that FN(+) matrix is essential for the generation of matrix filling for cavity spaces. There was a evidence that perivascular cells indicate NG2(+) pericytes, dorsal spinal cord hemi section was performed on Nestin-GFP/NG2-DsRed transgenic mice, number of NG2(+) pericytes significantly accumulated after injury suggesting that pericytes subpopulation can act as stem cell to produce fibrous tissue (Goritz et al., 2011). Another recent study demonstrated that blocking the pericyte regeneration resulted in the failure of fibrous connective tissue in the injury site (Birbrair et al., 2014). This data suggest that pericytes is another cellular source of fibrotic scar tissue. However, expression level of fibronectin to produce beneficial effects in axon regeneration in rat contusive injury model still remains to be elucidated.
MMPs are zinc-dependent endopeptidase, these ECM enzymes play multifunction. Basically, they involved in ECM degradation and tissue remodeling after injury. The specific role of MMPs in certain circumstance still be controversial. In acute phase of injury, MMPs may cause break down Blood Brain Barirer, edema and hemorrhage (Asahi et al., 2001). Howerver sufficient roles of these proteinase in chronic phages was not fully understood. There is increasing evidences that gelatinase activity has benifical role in matrix remodelling and wound healing (Hsu et al., 2006; Kyriakides et al., 2009). In a study,
MMP-29
9 increased 7 -14 days after stroke and colocolized with marker of neurovascular remodelling. Treatment of MMPs inhibitors at 7 days after stroke hindered neurovascular remodelling, enhanced ischemic injury and deteriorated functional recovery (Zhao et al., 2006). The activity and protein expression of MMP-9 exclusively in FN(+) matrix (Fig. 6A,H) suggested that I-5 may induce ECM remodeling via MMP-9 activity. MMP-9 level was elevated together with FN(+) deposition, they showed similar expression pattern at injury site, suggesting that MMP-9 activity may be required for fibronectin production. In order to investigate necessary relationship between FN(+) matrix and MMP-9, I planned to perform loss of function experiment. Particularly, mixing I-5 with MMP9 inhibitor to attenuate MMP-9 activity to test the hypothesis that MMP-9 is necessary for fibroblast accumulation. This experiment is one of the going on stuffs of my work.
Microglial cells play a role in phagocytosis by clearing debris due to necrosis, and secrete various factors and proteases with cytotoxicity on the neuronal tissue (Pan et al., 2002). Fortunately, popy(phosphazene) I-5 used in my study significantly suppressed inflammation while biomaterials used in previous studies did not obtain this feature, they need anti-inflammation agent such as minocycline (Kang et al., 2010) or Flavopiridol – an agent was documented to attenuate microglial activation (Ren et al., 2014). CD 45 positive blood borned macrophages also found to be subsided at 4-week time point. In addition, similar decline of CD68 positive macrophages was observed in I-5 injected animals 4 week after I-5 injection (data not shown). These results suggested that I-5 affected to almost all kind of macrophages. The suppression of macrophages by I-5 injection in my study can also be correlated with cavity reduction since the evidence of increased lesion cavity associated with high level of inflammation (Fitch et al., 1999). However the mechanism of action still not be addressed.
BBB scores demonstrated that the significant differences between animals with I-5 and PBS injection was seen at later time point 6th, 7th and 8th week (Fig. 8A), this result suggested that injury level was similar in all animals at early time point and that different behavior recovery was attributed to hydrogel effect. Coordination between fore- and hindlimbs is an important parameter that reflects quality locomotor function. It was significantly restored following I-5 injection, however the other parameters of footprints measurement were not affected by hydrogel injection. In many studies, hydrogel injection
30
alone did not bring out beneficial outcomes. Hydrogels are frequently mixed with trophic factors such as Neurotrophin 3 (NT3) (Piantino et al., 2006) or Fibroblast growth factor (FGF) (Chen et al., 2015; Kang et al., 2013), drugs such as liposomes (Nuttelman et al., 2006) or lipid microtubules (Schoenmakers et al., 2004). The behavior recovery was attributed to myelin protection effect of I-5. Following injury, myelin degeneration has occurred. This result in decreasing of myelin intensity. The data in this study showed that intensity of myelin in animals with I-5 injection was higher compared with that in PBS injected animals suggesting a protective role of I-5 injection.
Injectable hydrogel plays an important role in delivery method since drugs are not allowed to penetrate Blood Brain Barrier (BBB). While systematic delivery of drugs that cross BBB needs a large amount and by doing so usually causes unexpected side effects, local delivery directly to injury site carried by hydrogel would require very little amount with high efficiency. In this study, Taxol was successfully delivered by this method, proving effectiveness of the hydrogel as a drug carrier.
Another important application of injectable I-5 is carrying cells to injury site. Cell death occurred in host tissue by multiple mechanisms, survival rate is low between 0.2% and 10% (Kallur et al., 2006); (Bakshi et al., 2005). One of the most important reason is lacking the ECM for cell adherence (Frisch and Francis, 1994). In contrast with solid scaffolds, injectable gel has porous structure, high content of water facilitate cell distribution, adhesion and migration. This good point was proved via numerous studies, supporting cell survival and host tissue integration (Ballios et al., 2010). Increasing the survival of Schwan cells to over 36% (Patel et al., 2010). Enhanced differentiation of murine embryonic stem cells (Yang et al., 2015), neural stem cells (Tate et al., 2002); bone marrow stromal cells (Itosaka et al., 2009). Taking advantages of injectable hydrogel, I also tested I-5 has good effects in supporting neural stem cell survival, primary results have shown that I-5 enhanced viability of neural stem cell. In extending injectable hydrogel applications, other cell types such as induced pluripotent stem cells or mesenchymal stem cell also can be transplanted by this delivery method.
31
V. CONCLUSION
I demonstrated that injection of temperature sensitive I-5 alone successfully bridge lesion cavity in contusive SCI which close mimic to human injury. FN(+) matrix which is of perivascular fibroblasts is necessary for ECM generation. I also found that I-5 injection may induce ECM via MMP-9 activity, preserved myelin spare tissue and finally bring out functional recovery. Considering the facility of I-5 in a sol state mixing and delivering various drugs and cells, this approach can also be utilized as a versatile platform for multifaceted combinatorial therapy.
32
REFERENCES
1. Ahmed, Z., Underwood, S., and Brown, R.A. (2003). Nerve guide material made from fibronectin: assessment of in vitro properties. Tissue engineering 9, 219-231.
2. Asahi, M., Wang, X., Mori, T., Sumii, T., Jung, J.C., Moskowitz, M.A., Fini, M.E., and Lo, E.H. (2001). Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 7724-7732.
3. Austin, J.W., Kang, C.E., Baumann, M.D., DiDiodato, L., Satkunendrarajah, K., Wilson, J.R., Stanisz, G.J., Shoichet, M.S., and Fehlings, M.G. (2012). The effects of intrathecal injection of a hyaluronan-based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis. Biomaterials 33, 4555-4564.
4. Bakshi, A., Keck, C.A., Koshkin, V.S., LeBold, D.G., Siman, R., Snyder, E.Y., and McIntosh, T.K. (2005). Caspase-mediated cell death predominates following engraftment of neural progenitor cells into traumatically injured rat brain. Brain research 1065, 8-19.
5. Ballios, B.G., Cooke, M.J., van der Kooy, D., and Shoichet, M.S. (2010). A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials 31, 2555-2564. 6. Birbrair, A., Zhang, T., Files, D.C., Mannava, S., Smith, T., Wang, Z.M., Messi, M.L., Mintz,
A., and Delbono, O. (2014). Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem cell research & therapy 5, 122.
7. Burnside, E.R., and Bradbury, E.J. (2014). Manipulating the extracellular matrix and its role in brain and spinal cord plasticity and repair. Neuropathology and applied neurobiology 40, 26-59.
8. Chen, B., He, J., Yang, H., Zhang, Q., Zhang, L., Zhang, X., Xie, E., Liu, C., Zhang, R., Wang, Y., et al. (2015). Repair of spinal cord injury by implantation of bFGF-incorporated HEMA-MOETACL hydrogel in rats. Scientific reports 5, 9017.
9. Ek, C.J., Habgood, M.D., Callaway, J.K., Dennis, R., Dziegielewska, K.M., Johansson, P.A., Potter, A., Wheaton, B., and Saunders, N.R. (2010). Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PloS one 5, e12021.
33
10. El Waly, B., Macchi, M., Cayre, M., and Durbec, P. (2014). Oligodendrogenesis in the normal and pathological central nervous system. Frontiers in neuroscience 8, 145.
11. Fitch, M.T., Doller, C., Combs, C.K., Landreth, G.E., and Silver, J. (1999). Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. The Journal of neuroscience : the official journal of the Society for Neuroscience 19, 8182-8198.
12. Friedman, J.A., Windebank, A.J., Moore, M.J., Spinner, R.J., Currier, B.L., and Yaszemski, M.J. (2002). Biodegradable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery 51, 742-751; discussion 751-742.
13. Frisch, S.M., and Francis, H. (1994). Disruption of epithelial cell-matrix interactions induces apoptosis. The Journal of cell biology 124, 619-626.
14. Fujiki, M., Kobayashi, H., Inoue, R., and Goda, M. (2004). Electrical preconditioning attenuates progressive necrosis and cavitation following spinal cord injury. Journal of neurotrauma 21, 459-470.
15. Gao, M., Lu, P., Bednark, B., Lynam, D., Conner, J.M., Sakamoto, J., and Tuszynski, M.H. (2013). Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 34, 1529-1536.
16. Goritz, C., Dias, D.O., Tomilin, N., Barbacid, M., Shupliakov, O., and Frisen, J. (2011). A pericyte origin of spinal cord scar tissue. Science (New York, NY) 333, 238-242.
17. Hamers, F.P., Koopmans, G.C., and Joosten, E.A. (2006). CatWalk-assisted gait analysis in the assessment of spinal cord injury. Journal of neurotrauma 23, 537-548.
18. Hsu, J.Y., McKeon, R., Goussev, S., Werb, Z., Lee, J.U., Trivedi, A., and Noble-Haeusslein, L.J. (2006). Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 9841-9850.
19. Hwang, D.H., Shin, H.Y., Kwon, M.J., Choi, J.Y., Ryu, B.Y., and Kim, B.G. (2014). Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin-like growth factor-1 signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 12788-12800.
20. Itosaka, H., Kuroda, S., Shichinohe, H., Yasuda, H., Yano, S., Kamei, S., Kawamura, R., Hida, K., and Iwasaki, Y. (2009). Fibrin matrix provides a suitable scaffold for bone marrow
34
stromal cells transplanted into injured spinal cord: a novel material for CNS tissue engineering. Neuropathology : official journal of the Japanese Society of Neuropathology 29, 248-257.
21. Jeong, B., Kim, S.W., and Bae, Y.H. (2002). Thermosensitive sol-gel reversible hydrogels. Advanced drug delivery reviews 54, 37-51.
22. Kallur, T., Darsalia, V., Lindvall, O., and Kokaia, Z. (2006). Human fetal cortical and striatal neural stem cells generate region-specific neurons in vitro and differentiate extensively to neurons after intrastriatal transplantation in neonatal rats. Journal of neuroscience research 84, 1630-1644.
23. Kang, C.E., Baumann, M.D., Tator, C.H., and Shoichet, M.S. (2013). Localized and sustained delivery of fibroblast growth factor-2 from a nanoparticle-hydrogel composite for treatment of spinal cord injury. Cells, tissues, organs 197, 55-63.
24. Kang, Y.M., Hwang, D.H., Kim, B.G., Go, D.H., and Park, K.D. (2010). Thermosensitive Polymer-based Hydrogel Mixed with the Anti-inflammatory Agent Minocycline Induces Axonal Regeneration in Hemisected Spinal Cord. Macromol Res 18, 399-403.
25. King, V.R., Phillips, J.B., Hunt-Grubbe, H., Brown, R., and Priestley, J.V. (2006). Characterization of non-neuronal elements within fibronectin mats implanted into the damaged adult rat spinal cord. Biomaterials 27, 485-496.
26. Klouda, L., and Mikos, A.G. (2008). Thermoresponsive hydrogels in biomedical applications. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 68, 34-45.
27. Kretlow, J.D., Klouda, L., and Mikos, A.G. (2007). Injectable matrices and scaffolds for drug delivery in tissue engineering. Advanced drug delivery reviews 59, 263-273.
28. Kwok, J.C., Afshari, F., Garcia-Alias, G., and Fawcett, J.W. (2008). Proteoglycans in the central nervous system: plasticity, regeneration and their stimulation with chondroitinase ABC. Restorative neurology and neuroscience 26, 131-145.
29. Kyriakides, T.R., Wulsin, D., Skokos, E.A., Fleckman, P., Pirrone, A., Shipley, J.M., Senior, R.M., and Bornstein, P. (2009). Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix biology : journal of the International Society for Matrix Biology 28, 65-73.
35
30. Macaya, D., and Spector, M. (2012). Injectable hydrogel materials for spinal cord regeneration: a review. Biomedical materials (Bristol, England) 7, 012001.
31. Mahoney, M.J., and Anseth, K.S. (2006). Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials 27, 2265-2274.
32. Mothe, A.J., Tam, R.Y., Zahir, T., Tator, C.H., and Shoichet, M.S. (2013). Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials 34, 3775-3783.
33. Nomura, H., Zahir, T., Kim, H., Katayama, Y., Kulbatski, I., Morshead, C.M., Shoichet, M.S., and Tator, C.H. (2008). Extramedullary chitosan channels promote survival of transplanted neural stem and progenitor cells and create a tissue bridge after complete spinal cord transection. Tissue engineering Part A 14, 649-665.
34. Nuttelman, C.R., Tripodi, M.C., and Anseth, K.S. (2006). Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. Journal of biomedical materials research Part A 76, 183-195.
35. Paino, C.L., and Bunge, M.B. (1991). Induction of axon growth into Schwann cell implants grafted into lesioned adult rat spinal cord. Experimental neurology 114, 254-257.
36. Pan, J.Z., Ni, L., Sodhi, A., Aguanno, A., Young, W., and Hart, R.P. (2002). Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. Journal of neuroscience research 68, 315-322.
37. Patel, V., Joseph, G., Patel, A., Patel, S., Bustin, D., Mawson, D., Tuesta, L.M., Puentes, R., Ghosh, M., and Pearse, D.D. (2010). Suspension matrices for improved Schwann-cell survival after implantation into the injured rat spinal cord. Journal of neurotrauma 27, 789-801.
38. Piantino, J., Burdick, J.A., Goldberg, D., Langer, R., and Benowitz, L.I. (2006). An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Experimental neurology 201, 359-367. 39. Pradny, M., Michalek, J., Lesny, P., Hejcl, A., Vacik, J., Slouf, M., and Sykova, E. (2006).
Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 5: hydrolytically degradable materials. Journal of materials science Materials in medicine 17, 1357-1364. 40. Prang, P., Muller, R., Eljaouhari, A., Heckmann, K., Kunz, W., Weber, T., Faber, C.,
36
regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 27, 3560-3569.
41. Ren, H., Han, M., Zhou, J., Zheng, Z.F., Lu, P., Wang, J.J., Wang, J.Q., Mao, Q.J., Gao, J.Q., and Ouyang, H.W. (2014). Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials 35, 6585-6594.
42. Rooney, G.E., Endo, T., Ameenuddin, S., Chen, B., Vaishya, S., Gross, L., Schiefer, T.K., Currier, B.L., Spinner, R.J., Yaszemski, M.J., et al. (2009). Importance of the vasculature in cyst formation after spinal cord injury. Journal of neurosurgery Spine 11, 432-437.
43. Schoenmakers, R.G., van de Wetering, P., Elbert, D.L., and Hubbell, J.A. (2004). The effect of the linker on the hydrolysis rate of drug-linked ester bonds. Journal of controlled release : official journal of the Controlled Release Society 95, 291-300.
44. Soderblom, C., Luo, X., Blumenthal, E., Bray, E., Lyapichev, K., Ramos, J., Krishnan, V., Lai-Hsu, C., Park, K.K., Tsoulfas, P., et al. (2013). Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 13882-13887.
45. Sung, H.J., Meredith, C., Johnson, C., and Galis, Z.S. (2004). The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 25, 5735-5742.
46. Tabesh, H., Amoabediny, G., Nik, N.S., Heydari, M., Yosefifard, M., Siadat, S.O., and Mottaghy, K. (2009). The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochemistry international 54, 73-83.
47. Tate, M.C., Shear, D.A., Hoffman, S.W., Stein, D.G., Archer, D.R., and LaPlaca, M.C. (2002). Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell transplantation 11, 283-295. 48. Teng, Y.D., Lavik, E.B., Qu, X., Park, K.I., Ourednik, J., Zurakowski, D., Langer, R., and
Snyder, E.Y. (2002). Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proceedings of the National Academy of Sciences of the United States of America 99, 3024-3029.