0022-538X/08/$08.00
⫹0 doi:10.1128/JVI.02372-07
Copyright © 2008, American Society for Microbiology. All Rights Reserved.
Single Intranasal Immunization with Recombinant Adenovirus-Based
Vaccine Induces Protective Immunity against Respiratory
Syncytial Virus Infection
䌤
Jae-Rang Yu, Sol Kim, Jee-Boong Lee, and Jun Chang*
Division of Life and Pharmaceutical Sciences, Ewha Womans University, 11-1 Dae-Hyun Dong,
Seo-Dae-Mun Gu, Seoul 120-750, Korea
Received 2 November 2007/Accepted 6 December 2007
Respiratory syncytial virus (RSV) is a major cause of severe lower respiratory tract disease in infancy and
early childhood. Despite its importance as a pathogen, there is no licensed vaccine against RSV. The G
glycoprotein of RSV, a major attachment protein, is a potentially important target for protective antiviral
immune responses. Here, a recombinant replication-deficient adenovirus-based vaccine, rAd/3xG, expressing
the soluble core domain of G glycoprotein (amino acids 130 to 230) engineered by codon optimization and
tandem repetition for higher-level expression, was constructed and evaluated for its potential as an RSV
vaccine in a murine model. A single intranasal immunization with rAd/3xG provided potent protection against
RSV challenge which lasted for more than 10 weeks. Strong mucosal immunoglobulin A responses were also
induced by a single intranasal immunization but not by intramuscular or oral administration of rAd/3xG.
Interestingly, neither gamma interferon- nor interleukin-4-producing CD4 T cells directed to I-E
d-restricted
epitope were detected in the lungs of rAd/3xG-immune mice upon challenge, whereas priming with vaccinia
virus expressing RSV G (vvG) elicited strong Th1/Th2 mixed CD4 T-cell responses. Lung eosinophilia and
vaccine-induced weight loss were significantly lower in the rAd/3xG-immune group than in the vvG-primed
group. Together, our data demonstrate that a single intranasal administration of rAd/3xG elicits beneficial
protective immunity and represents a promising vaccine regimen against RSV infection.
Respiratory syncytial virus (RSV) is the most important viral
pathogen causing serious respiratory tract disease in infants
and young children worldwide. RSV is also receiving
increas-ing recognition as an important cause of lower respiratory tract
illness in immunocompromised patients and the elderly (13,
15, 16). Despite the importance of RSV as a respiratory
patho-gen, there is no licensed vaccine currently available against
RSV infection. Thus, developing an effective and safe RSV
vaccine remains a worldwide priority.
The RSV G glycoprotein was identified as the major RSV
attachment protein (24) and is thought to be important for
protection against RSV infection (39). G protein lacks any
major histocompatibility complex class I-restricted epitope (8,
26, 36) and has not yet been demonstrated to elicit a cytotoxic
T-lymphocyte response in either humans or mice (19, 29). It
has a single immunodominant I-E
depitope spanning amino
acids 183 to 198 and largely induces a specific subset of CD4 T
cells restricted to V
14 expression in the T-cell receptor (40,
42). Numerous studies have suggested that immunization with
RSV G is associated with the induction of polarized Th2-type
responses, which leads to pulmonary eosinophilia upon RSV
challenge of G-immunized mice (17, 20, 30, 35, 40). In
con-trast, it was recently suggested that G-specific immune
re-sponses are not solely the basis for vaccine-enhanced illness
and should not be excluded from potential vaccine strategies
(21, 22). In addition, intramuscular (i.m.) injection of plasmid
DNA encoding membrane G or secreted G induced balanced
Th1/Th2 immunity without an atypical pulmonary
inflamma-tion after RSV challenge in mice and cotton rats (3, 25),
suggesting that G protein may provide protection but not
in-duce enhanced lung pathology, depending on the vehicle
and/or method of delivery.
In the present study, we have targeted the RSV G protein
fragment between residues 130 and 230 and engineered the
sequence by codon optimization for optimal expression in
an-imal cells and by the addition of a secretory signal sequence
derived from tissue plasminogen activator (t-PA). Moreover,
this sequence was engineered to be multiple-copy tandem
re-peats in the same open reading frame for higher
immunoge-nicity (23, 31, 47). Replication-defective recombinant
adeno-virus (rAd) vaccines (rAd/1xG and rAd/3xG) were then
generated and evaluated for their potential as vaccines. We
show here that a single intranasal (i.n.) immunization of rAd/
3xG induces a strong serum immunoglobulin G (IgG)
re-sponse, a mucosal IgA rere-sponse, and long-term protection
following RSV challenge without vaccine-enhanced illness.
MATERIALS AND METHODS
Preparation of RSV stock.RSV strain A2 was propagated in HEp-2 cells (ATCC, Manassas, VA) in Dulbecco’s modified Eagle’s medium (Life Technol-ogies, Gaithersburg, MD) supplemented with 3% heat-inactivated fetal calf serum, 2 mM glutamine, 20 mM HEPES, nonessential amino acid, penicillin, and streptomycin and titrated for infectivity by plaque assay.
Construction of replication-defective rAds.A coding sequence of RSV G protein spanning amino acid residues 130 to 230 (RSV strain A2) was synthe-sized, in which codon substitutions were made for minimized usage of rare codons (Bioneer Corp., Daejeon, Korea). Codons for a six-histidine stretch as a
* Corresponding author. Mailing address: College of Pharmacy,
Ewha Womans University, 11-1 Dae-Hyun Dong, Seo-Dae-Mun Gu,
Seoul 120-750, Korea. Phone: 82-2-3277-2549. Fax: 82-2-3277-3051.
E-mail: tcell@ewha.ac.kr.
䌤
Published ahead of print on 19 December 2007.
2350
on March 16, 2017 by Ewha Woman`s University
http://jvi.asm.org/
tag and two consecutive stop codons were attached at 3⬘ terminus. This synthetic DNA was sequenced and then subcloned into pGEM-t-EASY vector (Promega, Madison, WI). The RSV G protein region was again amplified with various oligonucleotide primers containing appropriate restriction enzyme sites and jux-taposed three times with four glycine residues as a linker between each repeated region (Fig. 1). The original RSV G sequences were also cloned into the same sites of the vector. A start codon and the signal sequence of human t-PA were then inserted in the N termini of the RSV G fragments. Finally, the entire open reading frame was excised by KpnI/XhoI digestion and inserted into the same sites of pShuttle-CMV vector. Replication-defective Ads (serotype 5) were gen-erated by insertion of foreign sequences by homologous recombination and subsequent purification of recombinant progeny as described previously (18). Briefly, the shuttle vector plasmid was electroporated into electrocompetent BJ5183 cells carrying the pAdEasy-1 Ad genomic DNA. Recombinant Ad DNA was isolated and transfected into HEK293 cells to generate rAd/1xG and rAd/ 3xG viruses. Control Ad (rAd/control) was generated by the same method using the empty pShuttle-CMV vector. The recombinant viruses were amplified, pu-rified on CsCl2gradients, and titrated according to the protocols provided by the
manufacturer. The correct expression and secretion of RSV G protein fragments by rAd/3xG-infected HEp-2 cells were verified by Western blotting using G-specific monoclonal antibody (clone 131-2G; Chemicon International, Inc., Temecula, CA) and horseradish peroxidase-conjugated streptavidin (Zymed Laboratories, San Francisco, CA).
Immunization and challenge. Female BALB/c mice were purchased from Charles River Laboratories Inc. (Yokohama, Japan) and kept under specific-pathogen-free conditions. For immunization, 6- to 8-week-old mice were inoc-ulated with various doses of replication-defective Ad via the i.n., i.m., or oral route. For i.n. immunizations, mice were lightly anesthetized by ether/chloroform inhalation, and 5⫻ 107
PFU vaccine or control virus in a volume of 50l was applied to the left nostril. i.m. immunization was performed by injection of 5⫻ 107
PFU of vaccine in 100l into mouse hind limbs. For oral immunization, mice were deprived of water and food a few hours before injection and immunized with 5⫻ 107
PFU of virus in 200l of phosphate-buffered saline (PBS) by proximal esophageal intubation with a mouse feeding needle. Male and female pups were immunized with 3⫻ 107
PFU of rAd in volumes of 20 to 30l at 7 to 14 days of age. Vaccinia virus expressing RSV G (vvG) (5⫻ 106PFU) was
inoculated at the base of the tail by scarification (36). Three to four weeks later the mice were challenged i.n. with 3⫻ 106to 1⫻ 107PFU of live RSV A2 if
necessary. All animal studies were performed according to the guidelines of our Institutional Animal Care and Use Committee.
ELISA.Blood was obtained from the retro-orbital plexus with a heparinized capillary tube, collected in an Eppendorf tube, and centrifuged, and serum was stored at⫺20°C. RSV G protein-specific antibody titers in immunized mice were measured by a direct enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated overnight with 100l/well of 10-g/ml RSV A2 antigen (U.S. Biological, Swampscott, MA) or 2⫻ 104PFU of purified RSV A2
diluted in PBS and then blocked with PBS containing 2% bovine serum albumin and 0.05% Tween 20 for 2 h. Sera or lavage fluids were then added in serial dilutions and incubated for 2 h. The plates were washed five times with PBS containing 0.05% Tween 20 and incubated for 1 h with various dilutions of horseradish peroxidase-conjugated affinity-purified rabbit anti-mouse total IgG,
IgG1, IgG2a, or IgA secondary antibody (Zymed Laboratories, San Francisco, CA). The plates were washed three times and developed with 3,3⬘,5,5⬘-tetra-methylbenzidine, and the reaction was stopped with 1 M H3PO4and analyzed at
450 nm with a Thermo ELISA plate reader.
RSV titer in lung. Four days after RSV challenge, subsets of mice were euthanized and the lungs were removed into Eagle’s modified essential medium. The tissues were then processed through a steel screen to obtain a single-cell suspension, and particulate matter was removed by passage through a 70-m cell strainer (BD Labware, Franklin Lakes, NJ). The supernatants were collected, and RSV titers in the supernatants were measured by plaque assay on subcon-fluent HEp-2 monolayers. The data are expressed as the PFU per gram of lung tissue.
BAL.At 5 days postchallenge, a subset of mice from each group were sacrificed and tracheotomy was performed. The lung airways were washed with 0.8 ml of PBS containing 1% fetal bovine serum (FBS). The collected bronchoalveolar lavage (BAL) fluid cells and supernatants were used for counting eosinophils by hematoxylin-eosin staining and measuring secretory IgA titers, respectively.
Preparation of lung lymphocytes and flow cytometric analysis.The lungs were perfused with 5 ml of PBS containing 10 U/ml heparin (Sigma-Aldrich, St. Louis, MO) through the right ventricle using a syringe fitted with 25-gauge needle. The lungs were then removed and placed in RPMI medium supplemented with glutamine, gentamicin, penicillin G, and 10% FBS (HyClone, Logan, UT). The tissue was then processed through a steel screen to obtain a single-cell suspen-sion, and particulate matter was removed by passage through a 70-m Falcon cell strainer (BD Labware, Franklin Lakes, NJ). Freshly explanted BAL fluid or lung cells were purified by density gradient centrifugation and stained in PBS–3% FBS–0.09% NaN3using fluorochrome-conjugated antibodies. The antibodies
used were anti-CD3e (clone 145-2C11), anti-CD4 (clone RM4-5), anti-CD44 (clone IM7), anti-CD49d (clone R1-2), and anti-V14 TCR (clone 14-2). All antibodies were purchased from BD PharMingen (San Diego, CA). After stain-ing, cells were fixed in PBS–2% (wt/vol) paraformaldehyde, and events were acquired using a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA). To enumerate the number of cytokine-producing cells, intracellular cytokine staining was performed. In brief, 2⫻ 106
freshly explanted lung lymphocytes were cultured in a culture tube. Cells were left untreated or stimulated with 10 M G(183–195) peptide (WAICKRIPNKKPG) and then incubated for 5 h at 37°C in 5% CO2. Brefeldin A (5g/ml) (Sigma-Aldrich) was added for the
duration of the culture period to facilitate intracellular cytokine accumulation. Cells were then stained for surface markers, washed, fixed, permeabilized with fluorescence-activated cell sorter buffer containing 0.5% saponin (Sigma-Aldrich, Seoul, Korea), and stained for gamma interferon (IFN-␥). The antibod-ies used were anti-IFN-␥ (clone XMG1.2) or its control isotype antibody (rat IgG1). Dead cells were excluded on the basis of forward and side light scatter patterns. Data were collected using CELLQuest software (BD Biosciences) and analyzed with CELLQuest and WinMDI version 2.9 software (Scripps Research Institute, La Jolla, CA).
Data analysis.Comparison of differences was conducted by using an unpaired, two-tailed Student t test. The difference was considered statistically significant when the P value wasⱕ0.05.
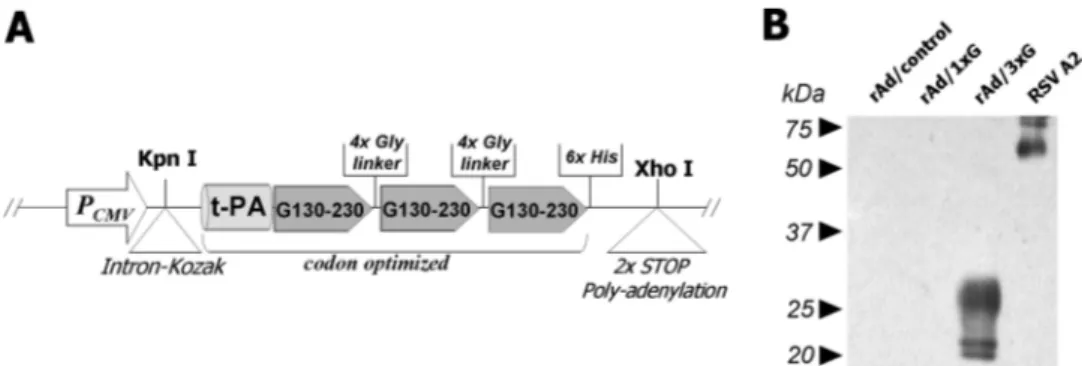
FIG. 1. Construction and characterization of rAd/3xG vaccine. (A) A shuttle vector was designed as shown in the diagram and constructed by
several cloning steps as described in Materials and Methods. The vector was used to generate the replication-deficient rAd/3xG by homologous
recombination with the Ad genomic DNA. (B) The expression of the recombinant RSV G protein fragment by rAd/3xG was confirmed by Western
blotting. Culture supernatants were prepared from HEp-2 cells infected with rAd/1xG, rAd/3xG, or rAd/control and subjected to Western blotting
with an anti-RSV glycoprotein monoclonal antibody. RSV A2-infected culture supernatant was used as a positive control.
V
OL. 82, 2008
PROTECTIVE IMMUNITY AGAINST RSV INDUCED BY rAd VACCINE
2351
on March 16, 2017 by Ewha Woman`s University
http://jvi.asm.org/
RESULTS
Construction of rAd expressing an RSV G protein fragment.
The strategy that we employed to enhance the vaccine
poten-tial the of RSV G protein fragment (amino acid residues 130 to
230) was to increase expression by codon optimization and
secretion of the recombinant protein by the addition of a signal
sequence from t-PA. In addition, three copies of this RSV G
fragment were tandemly repeated for higher immunogenicity
(23, 31, 47). Given the natural tropism of Ad, a
replication-defective rAd vector might be an ideal candidate for delivery of
an RSV vaccine. Thus, the engineered coding sequence was
placed into Ad DNA under the control of a cytomegalovirus
enhancer and promoter by homologous recombination,
result-ing in the generation of the replication-defective rAd rAd/3xG
(Fig. 1A). For comparison, rAd/1xG, containing a single copy
of the RSV G fragment, was used. Using a G-specific
mono-clonal antibody and immunoblotting, multiple bands of
ap-proximately 20 kDa to 30 kDa were detected only in the
cul-ture supernatant from HEp-2 cells infected with rAd/3xG and
not in supernatant from either rAd/control- or
rAd/1xG-in-fected HEp-2 cells (Fig. 1B). However, we have observed a
weak positive signal in an ELISA with an rAd/1xG-infected
sample, indicating a much lower level of G fragment
expres-sion by rAd/1xG (data not shown). These results indicate that
rAd/3xG successfully expresses the specific RSV G protein
fragment and that the level of expression was significantly
enhanced by use of our strategy.
Humoral immune response to rAd/3xG.
We next examined
whether rAd/3xG vaccine was capable of eliciting
antigen-spe-cific immune responses in vivo and, if so, what were the
char-acteristics of such immunity and which route was the best one.
BALB/c mice were inoculated once via the i.n., i.m., or oral
route with the same doses of rAd/1xG, rAd/3xG, or control Ad.
As a comparison, live RSV A2 was given once by i.n.
instilla-tion, and at 2 weeks after immunization total anti-RSV IgG
titers were determined by ELISA. Both i.n. and i.m.
immuni-zation with rAd/3xG induced strong total IgG responses in
serum even after a single injection, as demonstrated in Fig. 2A.
FIG. 2. Characterization of humoral immune responses induced by rAd/3xG. (A) BALB/c mice were immunized once with rAd (rAd/1xG,
rAd/3xG, or rAd/control) or with live RSV A2 via the indicated routes, and systemic anti-RSV IgG antibody titers were measured by serum ELISA
3 weeks after immunization. The results represent log
2end point values from four or five individual mice. (B) Average IgA titers in the BAL fluid
were measured 3 weeks after rAd/3xG immunization. Error bars indicate the standard deviation. (C) Total IgA titers in the supernatants of lung
homogenates were determined for each group 4 days after RSV challenge. (D) Fourteen-day-old pups were immunized with 3
⫻ 10
7PFU of
rAd/3xG or rAd/control in a volume of 20
l. Anti-RSV IgG antibody titers were determined 2 weeks later.
on March 16, 2017 by Ewha Woman`s University
http://jvi.asm.org/
Oral administration led to a relatively weaker RSV-specific
serum IgG response than i.n. or i.m. application. However, i.n.
immunization with the same dose of rAd/1xG failed to elicit
any detectable IgG response above the control value (Fig. 2A).
Notably, the levels of serum IgG responses in
rAd/3xG-immu-nized mice (i.n.) were comparable to those induced by live
RSV inoculation (i.n.) (Fig. 2A), indicating potent
immunoge-nicity of the rAd/3xG vaccine in vivo.
Secretory IgA is the first line of host defense against aerial
pathogens and appears to protect against infection of upper
respiratory tract (6). Thus, an effective RSV vaccine should
induce virus-specific secretory IgA preferably in the respiratory
mucosal area. To examine whether rAd/3xG vaccination elicits
an IgA response in the respiratory tract, BAL was performed
after immunization and levels of IgA were determined by
RSV-specific ELISA. As shown in Fig. 2B, the level of mucosal
IgA was much higher in the i.n. immunized group than in the
other groups when BAL fluid was tested at 21 days after
im-munization. Each group of mice immunized i.m. or orally with
rAd/3xG exhibited basal levels of IgA response. We also
checked the level of anti-RSV IgA in the lungs at 4 days after
RSV challenge, and rAd/3xG-immune and RSV-immune mice
showed significantly higher levels of IgA than control mice
(P
⬍ 0.01) (Fig. 2C). The difference between the
rAd/3xG-immune and RSV-rAd/3xG-immune mice was not statistically
signifi-cant, although the level was slightly higher in rAd/3xG group.
Next, we investigated whether immunization with rAd/3xG
also induces strong humoral responses in young pups or
neo-nates. The 14-day-old pups immunized with rAd/3xG showed
anti-RSV IgG responses (Fig. 2D), although the mean titer was
lower than that in the adult group (Fig. 2A). However, we
failed to detect an anti-RSV humoral response in 7- and
10-day-old pups or neonates (data not shown), possibly due to
immaturity of the immune system (1).
We next examined the effect of preexisting immunity against
the Ad vector. The mice were preexposed to rAd/control,
rested for 3 weeks, and then immunized with the rAd/3xG
vaccine. There was no significant difference in the anti-RSV
antibody response between the nonpreexposed group and the
group preexposed to 1
⫻ 10
7PFU (P
⫽ 0.11), although the
mean titer in the group preexposed to 1
⫻ 10
8PFU was
decreased (P
⬍ 0.05) (Fig. 3). More interestingly, the levels of
secreted anti-RSV IgA in BAL fluid were not significantly
changed among all groups of mice (Fig. 3), and all groups of
mice showed no detectable RSV replication in the lungs when
challenged with 2
⫻ 10
6PFU of RSV at 4 weeks after
immu-nization (data not shown). Taken together, our results suggest
that a single immunization with rAd/3xG vaccine via the i.n.
route is more efficient than that by other administration routes
in inducing both serum IgG and respiratory IgA and is
suffi-ciently immunogenic even in the presence of preexisting
anti-Ad immunity.
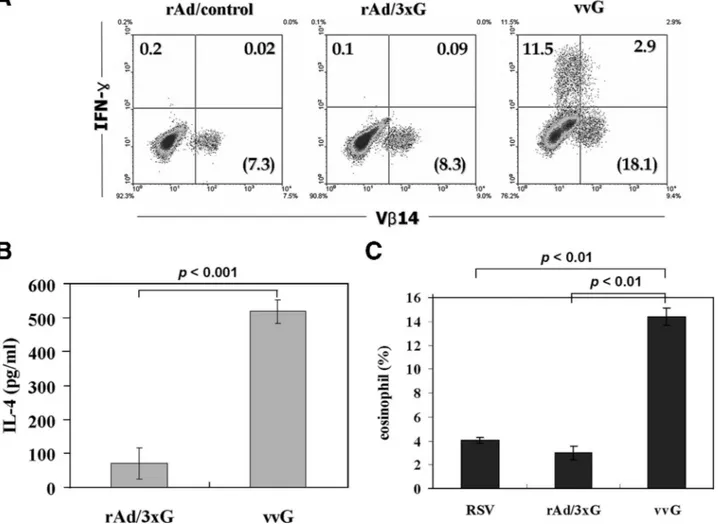
T-cell response to rAd/3xG.
It has been well demonstrated
that sensitization of mice with vvG primes G-specific CD4
T-cell responses and results in pulmonary eosinophilia
follow-ing RSV challenge (5, 17, 20, 30, 36). Since the G protein
fragment expressed by the rAd/3xG vaccine contains an I-E
d-restricted CD4 T-cell epitope (43), we examined whether rAd/
3xG immunization primes G-specific CD4 T cells. To this end,
rAd/3xG- or vvG-immune mice were challenged with RSV,
and 4 days after challenge lung lymphocytes were prepared,
stimulated with I-E
d-restricted G(183–195) epitope peptide,
and stained for IFN-
␥ production. As shown in Fig. 4A, a
G-specific CD4 T-cell response was barely detected in the
lungs of the rAd/3xG-immunized group (
ⱕ0.2% of lung CD4 T
cells), while a strong response (
ⱖ10% of lung CD4 T cells) was
observed in the vvG-immunized group by intracellular IFN-
␥
staining. We also checked the level of interleukin-4 (IL-4)
cytokine after in vitro stimulation of lung mononuclear cells
from both groups. As shown in Fig. 4B, in vitro-stimulated
cells from vvG-immune mice produced much more IL-4 than
those from rAd/3xG-immune mice. Taken together, these
re-sults clearly demonstrate that rAd/3xG immunization does not
prime a significant G-specific Th1/Th2 response, in contrast to
vvG scarification.
It was previously reported that the CD4 T-cell response
directed to the I-E
d-restricted immunodominant epitope
span-ning amino acids 183 to 195 of RSV G is sufficient to induce
enhanced disease after RSV challenge (40). Our results have
indicated that rAd/3xG immunization barely primes G-specific
CD4 T cells (Fig. 4A and B). Thus, it is quite unlikely that
rAd/3xG immunization would elicit vaccine-enhanced
eosino-philia after RSV challenge. To determine whether
immuniza-tion with rAd/3xG potentiates vaccine-enhanced
immuno-pathology, mice were challenged with RSV 3 weeks after
immunization and the levels of eosinophilia in BAL were
ex-amined at 5 days after challenge. As previously reported (36,
40), eosinophilia was markedly enhanced in vvG-immunized
animals (Fig. 4C) (15 to 25% of total BAL fluid cells).
How-ever, a weak infiltrate of eosinophils, ranging from 1 to 3% of
the total BAL fluid cells, was observed in mice previously
immunized with the rAd/3xG vaccine. This influx was similar in
magnitude to that observed in mice previously infected with
live RSV, suggesting that rAd/3xG hardly increases the risk of
development of eosinophil-related lung pathology.
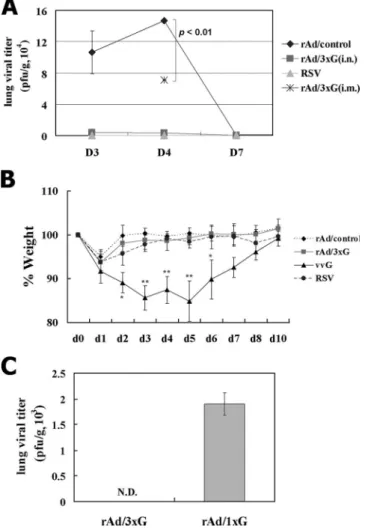
Protective efficacy of rAd/3xG against RSV challenge.
Hav-ing characterized the immune responses, we next wanted to
examine the immune protection against pulmonary RSV
chal-lenge conferred by mucosal and parenteral rAd/3xG
vaccina-FIG. 3. Immunogenicity of rAd/3xG in the presence of existing
vector immunity. The mice were first inoculated i.n. with various doses
of rAd/control as indicated and then after 3 weeks were immunized i.n.
with 5
⫻ 10
7PFU of rAd/3xG. Anti-RSV IgG antibody titers were
measured by serum ELISA 3 weeks later. Mucosal IgA titers in the
BAL fluid were also measured 3 weeks after immunization. OD,
op-tical density. Error bars indicate the standard deviation.
V
OL. 82, 2008
PROTECTIVE IMMUNITY AGAINST RSV INDUCED BY rAd VACCINE
2353
on March 16, 2017 by Ewha Woman`s University
http://jvi.asm.org/
tion. To this end, immune mice were challenged with live RSV
A2 at 4 weeks after immunization. While there was active RSV
replication in the lungs of the control mice, i.n. immunization
with rAd/3xG prevented any detectable RSV replication in the
lungs during the course of infection (Fig. 5A). A group of mice
previously infected with live RSV A2 also exhibited complete
protection against the challenge. However, i.m. immunization
with rAd/3xG resulted in partial protection at the peak of viral
replication (day 4 after challenge) (Fig. 5A). In keeping with
potent lung protection, there was no significant weight loss
upon RSV challenge (Fig. 5B) and no significant disease score
(data not shown) in rAd/3xG-immune mice compared to
vvG-scarified mice. This protective immunity conferred by rAd/3xG
immunization lasted for more than 10 weeks, since RSV
chal-lenge at 10 weeks after immunization resulted in no detectable
virus replication in the lung (Fig. 5C). Together, these results
suggest that a single mucosal rAd/3xG vaccination gives rise to
long-term protective immunity without priming of pathological
CD4 T cells and subsequent vaccine-enhanced disease.
DISCUSSION
In this study, we targeted an RSV G protein fragment as an
immunogen, expressed by replication-defective rAd, to
evalu-ate its vaccine potential. We chose this G protein fragment for
several reasons: (i) the G glycoprotein is one of the major
immunogens among viral proteins in both humans and rodents
(27), (ii) this region contains a conserved B-cell epitope of both
subgroups A and B (2, 37, 41, 43), and (iii) a subunit vaccine
containing this G protein fragment (9, 33) and a plasmid DNA
vaccine expressing secreted G protein (3, 25) were shown to
induced protective immunity in rodents without induction of
lung pathology. The evidence for the exact role of G in
vaccine-enhanced pathology is still contradictory (21, 28, 44). Thus, it
FIG. 4. G-specific CD4 T-cell response and lung eosinophilia in rAd/3xG-immune mice. (A) Mice were immunized with rAd i.n. or with vvG
by scarification and challenged with RSV 3 weeks after immunization. Lung mononuclear cells were prepared from the lungs of the same groups
of mice (n
⫽ 4) 4 days after challenge and stimulated with G(183–195) peptide in the presence of brefeldin A. Cells were stained for CD4, IFN-␥,
and T-cell receptor V
14 and analyzed by flow cytometry. Cells gated for CD4 expression are shown in each dot plot, and the percentages represent
the frequency of G-specific IFN-
␥-positive cells. The numbers in parentheses indicate the percentage of total V14
⫹cells among CD4
⫹cells. The
results are representative of two independent experiments. (B) Lung mononuclear cells were stimulated in vitro with plate-coated anti-CD3 for
48 h, and the levels of IL-4 in the culture supernatant were determined by sandwich ELISA. One representative from two separate experiments
is shown. (C) BAL was performed 5 days after challenge, cell pellets were differentially stained, and the number of eosinophils was counted. Data
represent means
⫾ standard deviations (n ⫽ 4). The results are representative of three independent experiments.
on March 16, 2017 by Ewha Woman`s University
http://jvi.asm.org/
is worthwhile to investigate the protective role of RSV
G-specific immunity elicited by a novel Ad-based vaccine. rAd
vectors have been used successfully and safely in many
preclin-ical and clinpreclin-ical settings (reviewed in reference 38).
Replica-tion-defective Ad is an attractive vaccine delivery vehicle since
it has intrinsic properties that activate both the innate and the
adaptive arms of the immune system (11). It has also been
shown to have very high transduction rate (48). Our results
clearly show that immunization with the rAd/3xG vaccine
in-duces strong serum and mucosal antibody responses after a
single dose without priming of potently immunopathogenic
CD4 T cells, which leads to long-term protection from
exper-imental RSV infection without vaccine-enhanced illness. It is
noteworthy that single-dose immunization with rAd/3xG is
suf-ficient for the induction of protective immunity, while three
immunizations with plasmid DNA encoding RSV G were
re-quired in the DNA vaccine regimen (25). This could be relative
advantage of rAd/3xG over other vaccine candidates,
consid-ering the fact that infant vaccinees must be protected from
infection as soon as possible after birth.
The exact mechanisms responsible for the lack of CD4 T-cell
priming and induction of beneficial and protective immunity by
rAd/3xG need further investigation. One possible explanation
could be based on the intrinsic properties of defective rAd
vaccines. A number of reports of studies using rAd-based
vac-cines describe similar findings that Ad vectors induce potent
transgene product-specific antibody and/or CD8 T-cell
re-sponses but no overly potent CD4 T-cell rere-sponses in
experi-mental animal systems (reviewed in reference 38). Second, it
has been proposed that RSV G has an immunomodulatory
effect on both innate and adaptive immunity (7, 32). For
ex-ample, Bukreyev et al. showed that the secreted form of G and
the cysteine-rich region have a positive modulatory effect on
cytotoxic T-lymphocyte function, which is independent of virus
titers and RSV-induced inflammation (7). Thus, it is possible
that secreted G protein fragments expressed by rAd/3xG affect
the priming environment in a way more favorable to beneficial
protective immunity. Together, our results strongly suggest
that RSV G protein could be successfully applied as a
protec-tive immunogen against RSV infection, if appropriate delivery
vectors such as recombinant Ad are employed.
Our study has also shown that the i.n. route of injection is
better than any other route tested with regard to protective
immunity induced by rAd/3xG. The higher levels of
virus-specific mucosal IgA and serum IgG raised by i.n.
immuniza-tion of rAd/3xG seem to be correlated with better protecimmuniza-tion
against RSV challenge (Fig. 2A and B and 5A). These results
are consistent with previous reports that stimulation and
main-tenance of local mucosal antibody may be important in the
generation of effective protection (12, 34). Since the infection
route for RSV is via the respiratory tract, mucosal delivery
might be critical for the development of protective immunity at
the site of infection. In addition, mucosal immunization has
been reported to play an important role in avoiding
interfer-ence from maternal antibodies against the Ad vector (4, 10).
One of the major concerns about Ad-vectored vaccines is that
the induction of immune responses to this common human
pathogen in neonates and young infants is impaired by
mater-nally transferred antibodies (45). We have shown that i.n.
im-munization with rAd/3xG is capable of inducing strong serum
IgG and mucosal IgA responses even in the presence of vector
immunity (Fig. 3). Previously, it has also been shown that
mucosally delivered Ad vectors expressing viral antigen were
capable of inducing neutralizing antibodies and protective
im-munity in neonatal mice even in the presence of maternal
antibodies against the vaccine carrier (46). These results
sug-gest that when delivered through i.n. route, the rAd/3xG
vac-cine might be effective in human populations in which the
majority have preexisting immunity to Ad serotype 5 as a result
of natural exposure. Furthermore, injection of rAd has been
shown to generally induce inflammatory reactions by releasing
proinflammatory cytokines such as IL-6, tumor necrosis factor
alpha, and IL-1 (14), which may compensate for the Th2 bias
FIG. 5. Immune protection from respiratory RSV challenge and
reduced weight loss by vaccination with rAd/3xG. (A) Each group of
immune mice was challenged with 1
⫻ 10
7PFU RSV at 4 weeks after
immunization, and the level of RSV replication in the lungs was
de-termined by plaque assay during the postchallenge periods. Results are
expressed as the means
⫾ standard errors of the means from four or
five mice for each time point per group. The limit of detection is 200
PFU/gram of lung. (B) The same groups of immune mice were
chal-lenged with RSV and then weighed each day. Results are expressed as
the means
⫾ standard errors of the means from five mice for each
group.
*
, P
⬍ 0.01;
**
, P
⬍ 0.001. (C) Immune mice were challenged
with 1
⫻ 10
6PFU RSV at 10 weeks after immunization, and the level
of RSV replication was determined at day 4 after infection. N.D., not
detected.
V
OL. 82, 2008
PROTECTIVE IMMUNITY AGAINST RSV INDUCED BY rAd VACCINE
2355
on March 16, 2017 by Ewha Woman`s University
http://jvi.asm.org/
of the immature immune system in neonates. In this regard,
delivery of the RSV G protein fragment by an Ad vector as a
neutralizing antigen might have some advantages over the use
of other vehicles, particularly as neonatal vaccines.
In summary, we have provided evidence that a
replication-deficient rAd-based RSV vaccine represents a promising
can-didate for an RSV vaccine. One of the major advantages
as-sociated with this RSV vaccine might be its effectiveness in
inducing long-term protective immunity with a single dose. It is
also possible that the level and/or duration of protection
in-duced by mucosal vaccination with our vaccine candidate could
be further enhanced when given with appropriate adjuvant(s).
We are currently investigating whether the use of various
doses, mixtures with various adjuvants, and/or multiple
admin-istrations of the immunogen by prime-boost regimens could
further improve the efficacy of our vaccine candidate.
ACKNOWLEDGMENTS
We acknowledge the technical support from Sung-A Kim, Min-Ha
Lee, and Ji-Hye Yoon in the completion of this work.
This work was supported by a Korea Research Foundation grant
funded by the Korean government (MOEHRD) (KRF-2006-E00130),
by grant R15-2006-020 from the NCRC program of MOST and
KOSEF through the Center for Cell Signaling and Drug Discovery
Research at Ewha Womans University, and by the Brain Korea 21
project.
REFERENCES
1. Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553–564.
2. Akerlind-Stopner, B., G. Utter, M. A. Mufson, C. Orvell, R. A. Lerner, and
E. Norrby.1990. A subgroup-specific antigenic site in the G protein of respiratory syncytial virus forms a disulfide-bonded loop. J. Virol. 64:5143– 5148.
3. Andersson, C., P. Liljestrom, S. Stahl, and U. F. Power. 2000. Protection against respiratory syncytial virus (RSV) elicited in mice by plasmid DNA immunisation encoding a secreted RSV G protein-derived antigen. FEMS Immunol. Med. Microbiol. 29:247–253.
4. Belyakov, I. M., B. Moss, W. Strober, and J. A. Berzofsky. 1999. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. USA 96: 4512–4517.
5. Bembridge, G. P., R. Garcia-Beato, J. A. Lopez, J. A. Melero, and G. Taylor. 1998. Subcellular site of expression and route of vaccination influence pul-monary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J. Immunol. 161:2473– 2480.
6. Brandtzaeg, P. 2003. Role of secretory antibodies in the defence against infections. Int. J. Med. Microbiol. 293:3–15.
7. Bukreyev, A., M. E. Serra, F. R. Laham, G. A. Melendi, S. R. Kleeberger,
P. L. Collins, and F. P. Polack.2006. The cysteine-rich region and secreted form of the attachment G glycoprotein of respiratory syncytial virus enhance the cytotoxic T-lymphocyte response despite lacking major histocompatibil-ity complex class I-restricted epitopes. J. Virol. 80:5854–5861.
8. Connors, M., A. B. Kulkarni, P. L. Collins, C. Y. Firestone, K. L. Holmes,
H. C. Morse III, and B. R. Murphy.1992. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8⫹T cells, while that induced by Vac-F or Vac-G recombinants is mediated by anti-bodies. J. Virol. 66:1277–1281.
9. Corvaia, N., P. Tournier, T. N. Nguyen, J. F. Haeuw, U. F. Power, H. Binz,
and C. Andreoni.1997. Challenge of BALB/c mice with respiratory syncytial virus does not enhance the Th2 pathway induced after immunization with a recombinant G fusion protein, BBG2NA, in aluminum hydroxide. J. Infect. Dis. 176:560–569.
10. Crowe, J. E., Jr. 2001. Influence of maternal antibodies on neonatal immu-nization against respiratory viruses. Clin. Infect. Dis. 33:1720–1727. 11. Ertl, H. C., and Z. Xiang. 1996. Novel vaccine approaches. J. Immunol.
156:3579–3582.
12. Etchart, N., B. Baaten, S. R. Andersen, L. Hyland, S. Y. Wong, and S. Hou. 2006. Intranasal immunisation with inactivated RSV and bacterial adjuvants induces mucosal protection and abrogates eosinophilia upon challenge. Eur. J. Immunol. 36:1136–1144.
13. Falsey, A. R. 1998. Respiratory syncytial virus infection in older persons. Vaccine 16:1775–1778.
14. Ginsberg, H. S., L. L. Moldawer, P. B. Sehgal, M. Redington, P. L. Kilian,
R. M. Chanock, and G. A. Prince.1991. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc. Natl. Acad. Sci. USA 88:1651–1655.
15. Glezen, W. P., S. B. Greenberg, R. L. Atmar, P. A. Piedra, and R. B. Couch. 2000. Impact of respiratory virus infections on persons with chronic under-lying conditions. JAMA 283:499–505.
16. Han, L. L., J. P. Alexander, and L. J. Anderson. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. In-fect. Dis. 179:25–30.
17. Hancock, G. E., D. J. Speelman, K. Heers, E. Bortell, J. Smith, and C. Cosco. 1996. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J. Virol. 70:7783–7791.
18. He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509–2514.
19. Heidema, J., G. J. de Bree, P. M. De Graaff, W. W. van Maren, P.
Hooger-hout, T. A. Out, J. L. Kimpen, and G. M. van Bleek.2004. Human CD8(⫹)
T cell responses against five newly identified respiratory syncytial virus-derived epitopes. J. Gen. Virol. 85:2365–2374.
20. Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and
B. S. Graham.1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosino-philia after RSV challenge. J. Virol. 72:2871–2880.
21. Johnson, T. R., M. N. Teng, P. L. Collins, and B. S. Graham. 2004. Respi-ratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J. Virol. 78:6024–6032.
22. Johnson, T. R., S. M. Varga, T. J. Braciale, and B. S. Graham. 2004. V14⫹
T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV. J. Virol. 78:8753–8760.
23. Kjerrulf, M., B. Lowenadler, C. Svanholm, and N. Lycke. 1997. Tandem repeats of T helper epitopes enhance immunogenicity of fusion proteins by promoting processing and presentation. Mol. Immunol. 34:599–608. 24. Levine, S., R. Klaiber-Franco, and P. R. Paradiso. 1987. Demonstration that
glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 68:2521–2524.
25. Li, X., S. Sambhara, C. X. Li, L. Ettorre, I. Switzer, G. Cates, O. James, M.
Parrington, R. Oomen, R. P. Du, and M. Klein.2000. Plasmid DNA encod-ing the respiratory syncytial virus G protein is a promisencod-ing vaccine candidate. Virology 269:54–65.
26. Martinez, I., J. Dopazo, and J. A. Melero. 1997. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermu-tation events for the generation of antigenic variants. J. Gen. Virol. 78:2419– 2429.
27. Murphy, B. R., S. L. Hall, A. B. Kulkarni, J. E. Crowe, Jr., P. L. Collins, M.
Connors, R. A. Karron, and R. M. Chanock.1994. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 32:13–36.
28. Murphy, B. R., A. Sotnikov, P. R. Paradiso, S. W. Hildreth, A. B. Jenson,
R. B. Baggs, L. Lawrence, J. J. Zubak, R. M. Chanock, J. A. Beeler, et al.
1989. Immunization of cotton rats with the fusion (F) and large (G) glyco-proteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine 7:533–540.
29. Nicholas, J. A., K. L. Rubino, M. E. Levely, E. G. Adams, and P. L. Collins. 1990. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J. Virol. 64:4232–4241.
30. Openshaw, P. J., S. L. Clarke, and F. M. Record. 1992. Pulmonary eosino-philic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 4:493–500.
31. Oscherwitz, J., M. E. Zeigler, T. E. Gribbin, and K. B. Cease. 1999. A V3 loop haptenic peptide sequence, when tandemly repeated, enhances immu-nogenicity by facilitating helper T-cell responses to a covalently linked car-rier protein. Vaccine 17:2392–2399.
32. Polack, F. P., P. M. Irusta, S. J. Hoffman, M. P. Schiatti, G. A. Melendi,
M. F. Delgado, F. R. Laham, B. Thumar, R. M. Hendry, J. A. Melero, R. A. Karron, P. L. Collins, and S. R. Kleeberger.2005. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc. Natl. Acad. Sci. USA 102:8996– 9001.
33. Power, U. F., H. Plotnicky-Gilquin, T. Huss, A. Robert, M. Trudel, S. Stahl,
M. Uhlen, T. N. Nguyen, and H. Binz.1997. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fu-sion protein containing a respiratory syncytial virus G protein fragment. Virology 230:155–166.
34. Singleton, R., N. Etchart, S. Hou, and L. Hyland. 2003. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus
on March 16, 2017 by Ewha Woman`s University
http://jvi.asm.org/
tion in mice correlates with ineffective nasal antibody responses. J. Virol.
77:11303–11311.
35. Sparer, T. E., S. Matthews, T. Hussell, A. J. Rae, B. Garcia-Barreno, J. A.
Melero, and P. J. Openshaw.1998. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J. Exp. Med. 187:1921–1926. 36. Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific CD8⫹ T
lym-phocytes downregulate T helper cell type 2 cytokine secretion and pulmo-nary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421–432.
37. Sullender, W. M., K. Anderson, and G. W. Wertz. 1990. The respiratory syncytial virus subgroup B attachment glycoprotein: analysis of sequence, expression from a recombinant vector, and evaluation as an immunogen against homologous and heterologous subgroup virus challenge. Virology
178:195–203.
38. Tatsis, N., and H. C. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther.
10:616–629.
39. Taylor, G., E. J. Stott, M. Bew, B. F. Fernie, P. J. Cote, A. P. Collins, M.
Hughes, and J. Jebbett.1984. Monoclonal antibodies protect against respi-ratory syncytial virus infection in mice. Immunology 52:137–142. 40. Tebbey, P. W., M. Hagen, and G. E. Hancock. 1998. Atypical pulmonary
eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 188:1967–1972. 41. Trudel, M., F. Nadon, C. Seguin, and H. Binz. 1991. Protection of BALB/c
mice from respiratory syncytial virus infection by immunization with a syn-thetic peptide derived from the G glycoprotein. Virology 185:749–757.
42. Varga, S. M., X. Wang, R. M. Welsh, and T. J. Braciale. 2001. Immuno-pathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(⫹) T cells. Immunity 15:637–646.
43. Varga, S. M., E. L. Wissinger, and T. J. Braciale. 2000. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4⫹ T cell responses. J. Immunol.
165:6487–6495.
44. Vaux-Peretz, F., J. M. Chapsal, and B. Meignier. 1992. Comparison of the ability of formalin-inactivated respiratory syncytial virus, immunopurified F, G and N proteins and cell lysate to enhance pulmonary changes in Balb/c mice. Vaccine 10:113–118.
45. Wang, Y., Z. Xiang, S. Pasquini, and H. C. Ertl. 1997. Immune response to neonatal genetic immunization. Virology 228:278–284.
46. Xiang, Z., Y. Li, G. Gao, J. M. Wilson, and H. C. Ertl. 2003. Mucosally delivered E1-deleted adenoviral vaccine carriers induce transgene prod-uct-specific antibody responses in neonatal mice. J. Immunol. 171:4287– 4293.
47. Yankai, Z., Y. Rong, H. Yi, L. Wentao, C. Rongyue, Y. Ming, L. Taiming, L.
Jingjing, and W. Jie.2006. Ten tandem repeats of beta-hCG 109–118 en-hance immunogenicity and anti-tumor effects of beta-hCG C-terminal pep-tide carried by mycobacterial heat-shock protein HSP65. Biochem. Biophys. Res. Commun. 345:1365–1371.
48. Zhong, L., A. Granelli-Piperno, Y. Choi, and R. M. Steinman. 1999. Recom-binant adenovirus is an efficient and non-perturbing genetic vector for hu-man dendritic cells. Eur. J. Immunol. 29:964–972.