저작자표시 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. l 이 저작물을 영리 목적으로 이용할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다.
5
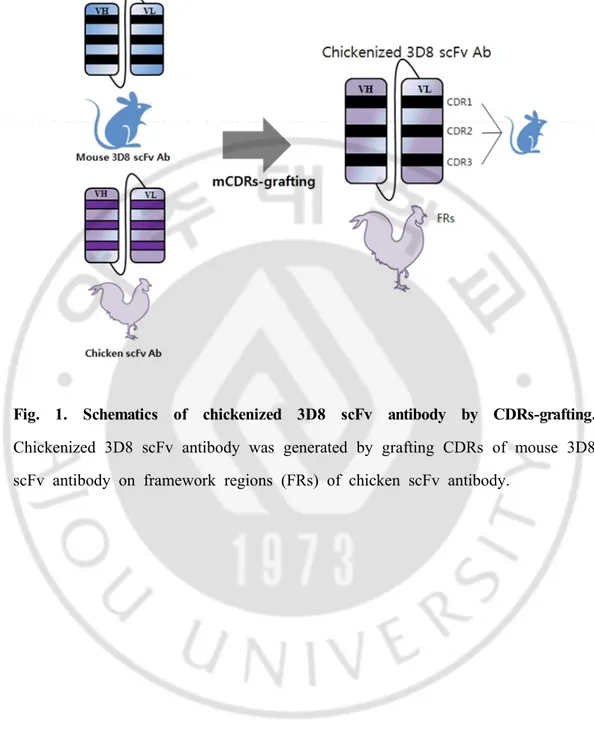
-Fig. 1. Schematics of chickenized 3D8 scFv antibody by CDRs-grafting. Chickenized 3D8 scFv antibody was generated by grafting CDRs of mouse 3D8 scFv antibody on framework regions (FRs) of chicken scFv antibody.
μ μ
β
μ
7
-μ
μ
μ
μ
9 -μ
μ
μ μ μ μ μ μ μ μ β
11
-μ
μ
μ
Fig. 2. Schedule of chicken immunization. Two groups of 2 female 28-week-old single comb white Leghorn (SCWL) chickens were immunized with 200 µl of the purified m3D8 scFv or CK6 protein. The proteins were mixed with complete Freund's Adjuvants (CFA, Sigma) for primary injection, followed by boosters at 2, 4, and 5 weeks with incomplete Freund's Adjuvants (IFA, Sigma). For each injection, 150 µg of proteins was diluted in sterile PBS to a volume of 100 µl and mixed with an equal volume of adjuvant. Chickens were inoculated on two sites, subcutaneously with 100 µl on the leg muscle and intramuscularly with 100 µl on the back of the neck. Sera were collected from individual chickens prior to 1st immunization and after 4th immunization.
-Fig. 3. Amino acid alignment of VHs and VLs from ten chicken antibodies. cAb-1 and cAb-2 were chicken antibodies against Eimeria tenella sporozoites. cAb-3~cAb-10 were chicken antibodies specific for infectious bursal disease virus.
15
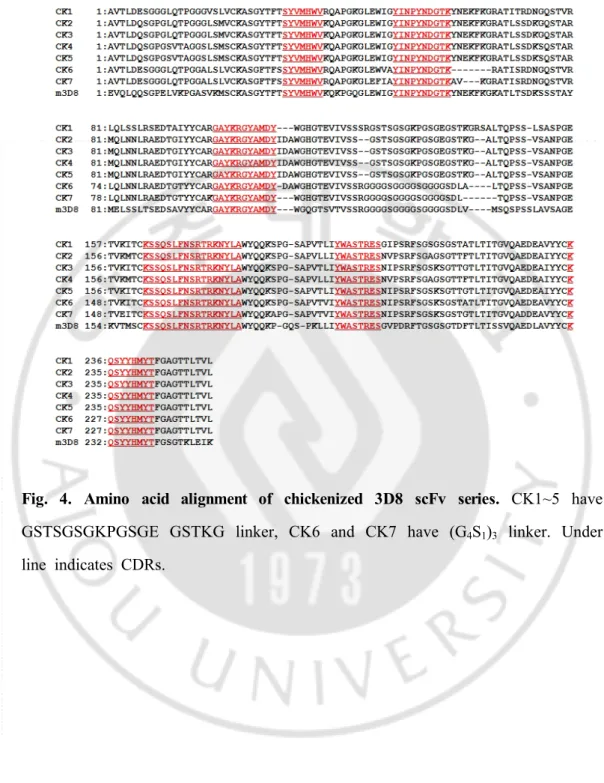
-Fig. 4. Amino acid alignment of chickenized 3D8 scFv series. CK1~5 have
GSTSGSGKPGSGE GSTKG linker, CK6 and CK7 have (G4S1)3 linker. Under
17
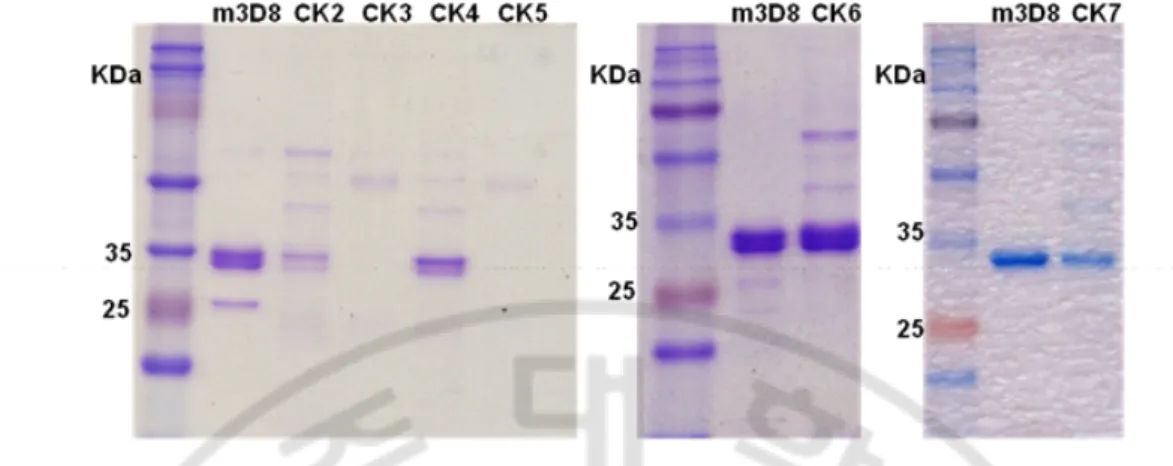
-Fig. 5. SDS-PAGE of the purified scFvs. Proteins (5 μg) were separated on 12 % acrylamide gels under denature condition and visualized with Coomassie Blue.
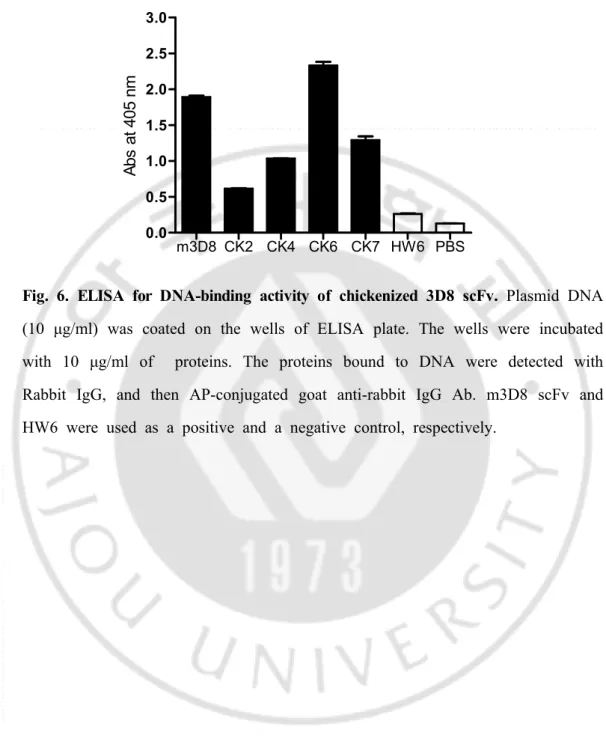
19 -m3D8 CK2 CK4 CK6 CK7 HW6 PBS 0.0 0.5 1.0 1.5 2.0 2.5 3.0 A bs a t 4 05 n m
Fig. 6. ELISA for DNA-binding activity of chickenized 3D8 scFv. Plasmid DNA (10 g/ml) was coated on the wells of ELISA plate. The wells were incubated μ
with 10 g/ml of proteins. The proteins bound to DNA were detected with μ
Rabbit IgG, and then AP-conjugated goat anti-rabbit IgG Ab. m3D8 scFv and HW6 were used as a positive and a negative control, respectively.
21
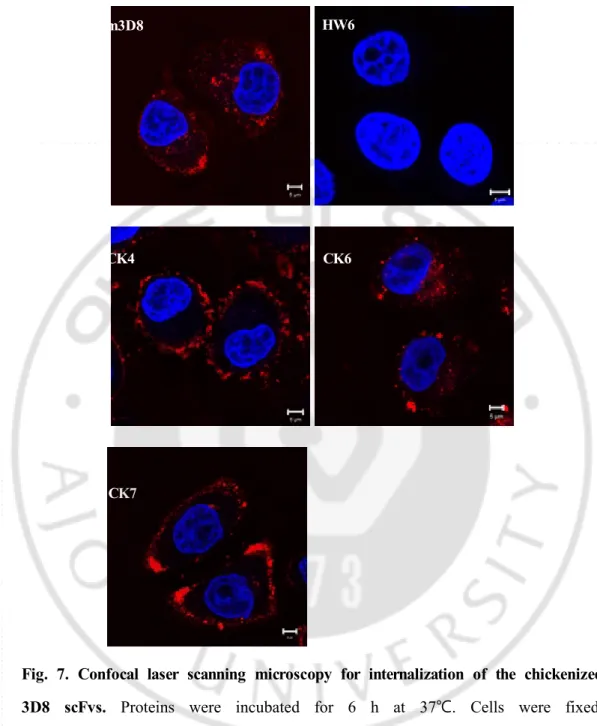
-Fig. 7. Confocal laser scanning microscopy for internalization of the chickenized 3D8 scFvs. Proteins were incubated for 6 h at 37℃. Cells were fixed, permeablized, and incubated with rabbit IgG and TRITC-anti-rabbit IgG. m3D8 scFv and HW6 were used as positive and negative control, respectively. Scale bar = 5 m.μ
m3D8 HW6
CK4 CK6
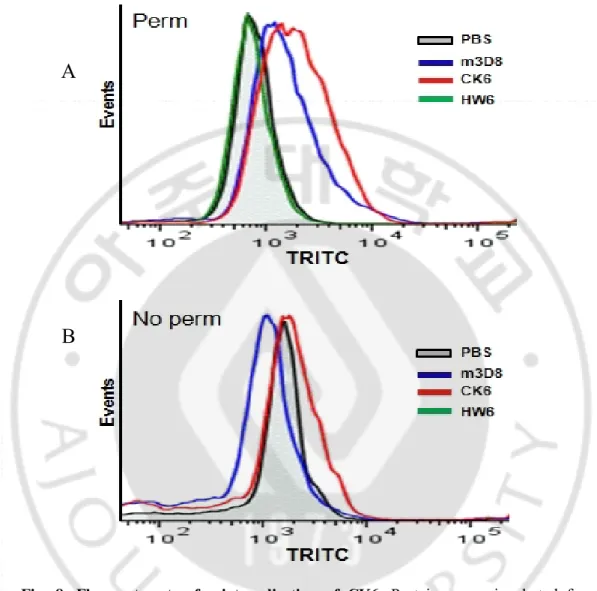
Fig. 8. Flow cytometry for internalization of CK6. Proteins were incubated for 6 h at 37℃. Cells were fixed and then either permeablized (A) or not (B). Cells were incubated with rabbit IgG and alexa fluor 647-goat anti-rabbit IgG, prior to flow cytometric analysis. m3D8 scFv and HW6 were used as positive and negative control, respectively.
B
A
-0 3-0 6-0 9-0 12-0 15-0 18-0 21-0 24-0 27-0 3-0-0 33-0 36-0 0 100 200 300 m3D8 m3D8+hp HW6 HW6+hp CK6 CK6+hp DNaseI DNaseI+hp Buffer Time (min) R F U
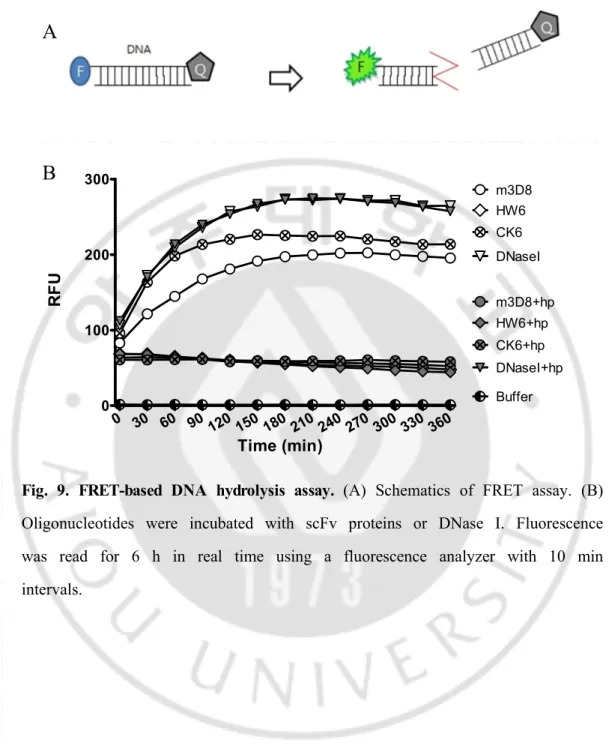
Fig. 9. FRET-based DNA hydrolysis assay. (A) Schematics of FRET assay. (B) Oligonucleotides were incubated with scFv proteins or DNase I. Fluorescence was read for 6 h in real time using a fluorescence analyzer with 10 min intervals.
B
A
25 -Table 1. Characteristics of the chickenized scFvs
scFv (mg/L)Yield DNA-binding% a DNA-hydrolysis% b internalizationCellular c
m3D8 5-10 100 100 55 CK1 0 NDd ND ND CK2 0-0.1 20 ND ND CK3 0 ND ND ND CK4 0.5-0.8 45 0 0 CK5 0.0 ND ND ND CK6 0.6 120 97 60 CK7 0.6 55 58 0
a Data are presented as relative binding activity to the m3D8 and represent the average values of three independent ELISA performed in triplicate wells.
b Data are presented as relative DNA-hydrolysis activity to the m3D8 and
represent the average values of three independent ELISA experiments performed in duplicate wells.
c The percentage of positive cells for internalized scFv was assessed by three independent flow cytometry.
-Fig. 10. SDS-PAGE of the purified scFvs without Protein A. Proteins (5 μg) were separated on 12 % acrylamide gels under denature condition and visualized with Coomassie Blue.
29
-A
B
C
Fig. 11. ELISA for immunogenicity of m3D8 scFv and CK6 in chicken. (A) m3D8 scFv without Protein A was coated on the well. Serum immunized with m3D8 scFv was added on the well. (B) CK6 without Protein A was coated on the well. Serum immunized with CK6 was added on the well. (C) m3D8 scFv without Protein A was coated on the well. Serum immunized with CK6 was added on the well. (D) CK6 without Protein A was coated on the well. Serum immunized with m3D8 scFv was added on the well. The serum IgY bound to antigen was detected with AP-conjugated goat anti-chicken IgY.
31
-Table 2. Comparison of IgY responses in the chickens immunized with scFv proteins Antigen used for well-coating Antigen used for immunizatio n a Relative absorbance of immuneserum to pre-serumb Chicken 1 Chicken 2 10-3 c 10-4 10-5 10-3 10-4 10-5 m3D8 m3D8 2.5 3.6 1.9 7.2 4.5 1.8 CK6 CK6 2.2 3.1 1.8 5.5 3.5 1.8 m3D8 CK6 4.1 2.2 1.3 3.9 1.7 1.2 CK6 m3D8 1.2 1.1 1.1 2.2 1.3 1.1
a Sera obtained from chicken immunized 4times with these scFv were used for this ELISA.
b Ratio of immune serum absorbance at 405 nm to preimmune serum absor
bance at 405 nm. Data are the average values of two independent ELISA experiments performed in duplicate wells.
35
-1. Abi-Ghanem D, Waghela SD, Caldwell DJ, Danforth HD, Berghman
LR: Phage display selection and characterization of single-chain recombinant antibodies against Eimeria tenella sporozoites. Vet Immunol Immunopathol 121: 58-67, 2008
2. Borras L, Gunde T, Tietz J, Bauer U, Hulmann-Cottier V, Grimshaw
JP, Urech DM: Generic approach for the generation of stable humanized single-chain Fv fragments from rabbit monoclonal antibodies. J Biol Chem 285: 9054-9066, 2010
3. Carter P: Improving the efficacy of antibody-based cancer therapies. Nat
Rev Cancer 1: 118-129, 2001
4. Dubrovskaya VV, Andryushkova AS, Kuznetsova IA, Toporkova LB,
Buneva VN, Orlovskaya IA, Nevinsky GA: DNA-hydrolyzing antibodies from sera of autoimmune-prone MRL/MpJ-lpr mice. Biochemistry (Mosc) 68: 1081-1088, 2003
5. Espert L, Degols G, Gongora C, Blondel D, Williams BR, Silverman
RH, Mechti N: ISG20, a new interferon-induced RNase specific for single-stranded RNA, defines an alternative antiviral pathway against RNA genomic viruses. J Biol Chem 278: 16151-16158, 2003
6. Foged C, Nielsen HM: Cell-penetrating peptides for drug delivery across
membrane barriers. Expert Opin Drug Deliv 5: 105-117, 2008
7. Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ: The
safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9: 325-338, 2010
8. Herron JN, He XM, Ballard DW, Blier PR, Pace PE, Bothwell AL, Voss EW, Jr., Edmundson AB: An autoantibody to single-stranded DNA: comparison of the three-dimensional structures of the unliganded Fab and a deoxynucleotide-Fab complex. Proteins 11: 159-175, 1991
9. Jang JY, Jeong JG, Jun HR, Lee SC, Kim JS, Kim YS, Kwon MH: A
nucleic acid-hydrolyzing antibody penetrates into cells via
caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell Mol Life Sci 66: 1985-1997, 2009
10. Jun HR, Pham CD, Lim SI, Lee SC, Kim YS, Park S, Kwon MH: An
RNA-hydrolyzing recombinant antibody exhibits an antiviral activity against classical swine fever virus. Biochem Biophys Res Commun 395: 484-489, 2010
11. Kashmiri SV, De Pascalis R, Gonzales NR, Schlom J: SDR grafting--a
new approach to antibody humanization. Methods 36: 25-34, 2005
12. Kim A, Lee JY, Byun SJ, Kwon MH, Kim YS: Viral genome RNA
degradation by sequence-selective, nucleic-acid hydrolyzing antibody inhibits the replication of influenza H9N2 virus without significant cytotoxicity to host cells. Antiviral Res 94: 157-167, 2012
13. Kim YR, Kim JS, Lee SH, Lee WR, Sohn JN, Chung YC, Shim HK,
Lee SC, Kwon MH, Kim YS: Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded DNAs without sequence specificity. J Biol Chem 281: 15287-15295, 2006
37
-antibody of predefined specificity. Nature 256: 495-497, 1975
15. Kwon MH, Lee MS, Kim KH, Park S, Shin HJ, Jang YJ, Kim HI:
Production and characterization of an anti-idiotypic single chain Fv that recognizes an anti-DNA antibody. Immunol Invest 31: 205-218, 2002
16. Lee G, Shim H-K, Kwon M-H, Son S-H, Kim K-Y, Park E-Y, Yang
J-K, Lee T-K, Auh C-K, Kim D, Kim Y-S, Lee S: RNA virus accumulation is inhibited by ribonuclease activity of 3D8 scFv in transgenic Nicotiana tabacum. Plant Cell, Tissue and Organ Culture (PCTOC), 2013
17. Mayor S, Pagano RE: Pathways of clathrin-independent endocytosis. Nat
Rev Mol Cell Biol 8: 603-612, 2007
18. Nevinsky GA, Kanyshkova TG, Buneva VN: Natural catalytic antibodies
(abzymes) in normalcy and pathology. Biochemistry (Mosc) 65: 1245-1255, 2000
19. Nishibori N, Horiuchi H, Furusawa S, Matsuda H: Humanization of
chicken monoclonal antibody using phage-display system. Mol Immunol 43: 634-642, 2006
20. Park KJ, Lee SH, Kim TI, Lee HW, Lee CH, Kim EH, Jang JY, Choi
KS, Kwon MH, Kim YS: A human scFv antibody against TRAIL receptor 2 induces autophagic cell death in both TRAIL-sensitive and TRAIL-resistant cancer cells. Cancer Res 67: 7327-7334, 2007
21. Parton RG, Simons K: The multiple faces of caveolae. Nat Rev Mol
Cell Biol 8: 185-194, 2007
Neumann RD, Larson SM: Anti-murine antibody response to mouse monoclonal antibodies: clinical findings and implications. Int J Rad Appl Instrum B 16: 121-125, 1989
23. Riechmann L, Muyldermans S: Single domain antibodies: comparison of
camel VH and camelised human VH domains. J Immunol Methods 231: 25-38, 1999
24. Sapats SI, Trinidad L, Gould G, Heine HG, van den Berg TP,
Eterradossi N, Jackwood D, Parede L, Toquin D, Ignjatovic J: Chicken recombinant antibodies specific for very virulent infectious bursal disease virus. Arch Virol 151: 1551-1566, 2006
25. Saxena SK, Gravell M, Wu YN, Mikulski SM, Shogen K, Ardelt W,
Youle RJ: Inhibition of HIV-1 production and selective degradation of viral RNA by an amphibian ribonuclease. J Biol Chem 271: 20783-20788, 1996
26. Seccamani E, Tattanelli M, Mariani M, Spranzi E, Scassellati GA,
Siccardi AG: A simple qualitative determination of human antibodies to murine immunoglobulins (HAMA) in serum samples. Int J Rad Appl Instrum B 16: 167-170, 1989
27. Seddiki N, Nato F, Lafaye P, Amoura Z, Piette JC, Mazie JC:
Calreticulin, a potential cell surface receptor involved in cell penetration of anti-DNA antibodies. J Immunol 166: 6423-6429, 2001
28. Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y: Autoantibody explosion
in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum 34: 501-537, 2004
39
-29. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF,
Schaller JG, Talal N, Winchester RJ: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25: 1271-1277, 1982
30. Vincke C, Loris R, Saerens D, Martinez-Rodriguez S, Muyldermans S,
Conrath K: General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem 284: 3273-3284, 2009
31. Yanase K, Smith RM, Puccetti A, Jarett L, Madaio MP:
Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J Clin Invest 100: 25-31, 1997
32. Zack DJ, Stempniak M, Wong AL, Taylor C, Weisbart RH:
Mechanisms of cellular penetration and nuclear localization of an anti-double strand DNA autoantibody. J Immunol 157: 2082-2088, 1996
-ABSTRACT-Generation of chickenized 3D8 scFv antibody from mouse
monoclonal anti-DNA antibody by CDRs-grafting
Jooho Roh
Department of Biomedical Sciences The Graduate School, Ajou University
(Supervised by Associate Professor Myung-Hee Kwon)
3D8 single chain variable fragment (scFv) derived from a MRL-lpr/lpr mouse is a catalyric anti nucleic acid antibody that has ability of cellular internalization and DNA/RNA-hydrolyzing activity. It has been reported that cells treated with 3D8 scFv protein showed the down-regulation against replication of RNA-genomed viruses and this anti-viral activity should be due to the cellular internalizaing and DNA/RNA- hydrolyzing activities of 3D8 scFv. To extend the possible applications of anti-viral activity of 3D8 scFv, we have developed a chickenized antibody of 3D8 scFv by CDRs (complementarity-derermining regions) grafting. A chickenized 3D8 scFv variant (CK) was generated by transferring six CDRs of 3D8 scFv between FRs (framework regions) of a chicken antibody which reveals high solubility and expression level in bacterial culture. CK6 of chickenized 3D8 scFv variant (CK1~7) retained the DNA-binding, DNA-hydrolysis, and cellular internalizing activities similar to that
41
-of the original mouse 3D8 scFv (m3D8). Our results demonstrate that chckenized 3D8 scFv can be generated by grafting CDRs.
Keyword: 3D8 scFv, anti nucleic acid antibody, DNA/RNA hydrolyzing activity, chickenization, CDR-grafting, Immunogenicity.