The Effects of Onion and Yuzu Extracts against
Ischemia/Hypoxia-Induced Dysfunction of
Blood-Brain Barrier
by
Soo-Wang Hyun
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
The Effects of Onion and Yuzu Extracts against
Ischemia/Hypoxia-Induced Dysfunction of
Blood-Brain Barrier
by
Soo-Wang Hyun
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for
The Degree of Master of Biomedical Sciences
Supervised by
Soo Hwan Lee, Ph.D.
Yi-Sook Jung, Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Soo
—
Wang Hyun is approved●
The Graduate
Decelnber,
SUPERVISORY COMM工
TTEE
Schoo1,
서
ou
19th, 2014
i
- ABSTRACT-
The Effects of Onion and Yuzu Extracts against Ischemia/Hypoxia
-Induced Dysfunction of Blood-Brain Barrier
This study investigated the potential beneficial effects of natural products and their major component on redistribution of ZO-1 and FoxO3a-mediated degradation of claudin-5 during ischemia/hypoxia using the mouse middle cerebral artery occlusion (MCAO) model and hypoxia model. The possible underlying mechanisms are also investigated, especially those linked to the oxidative stresses.
Onion extract (OE) and its major component, quercetin (Quer), as well as yuzu extract (YE) and hesperidin (HSP) prevented brain ischemia-induced brain edema in dose-dependent manners. Evans blue extravasation in the ischemic hemisphere of the mouse brain was also significantly reduced by treatment with the four materials. In addition, they inhibited the immunoreactivity of tight junction proteins (TJs; ZO-1 (Zonula occludens-1) and claudin-5).
In in vitro model, pre-treatment of OE, Quer, YE, and HSP significantly attenuated hypoxia-induced Blood-Brain Barrier (BBB) hyperpermeability. The four materials also inhibited hypoxia-induced redistribution of ZO-1, degradation of claudin-5, increase of matrix metalloproteinase (MMP)-3/9 mRNA levels, and translocation of Forkhead box O 3a (FoxO3a) into nucleus. The effects of antioxidant, trolox, and N-acetyl-cysteine (NAC) as well as siFoxO3a transfection also mimicked those effects of OE, Quer, YE, and HSP.
ii
However, transfection of siFoxO3a did not inhibit transcription of MMP-9 and redistribution of ZO-1 induced by hypoxia. In addition, OE, Quer, YE, and HSP attenuated the generation of oxidative stress induced by hypoxia, indicating that they may have antioxidant effects against hypoxia induced-dysfunction of BBB.
The results from this study demonstrate that OE, Quer, YE, and HSP prevent BBB hyperpermeability and tight junction proteins (TJs) disruption in MCAO and hypoxia model. In addition, these findings suggest that BBB protection by the four materials involves reduction of MMPs transcription, the inhibition of ZO-1 redistribution, and FoxO3a inhibition-mediated suppression of claudin-5 degradation, possibly through its antioxidant effects in hypoxia model.
Key words: Blood-brain barrier; Cerebral ischemia/Hypoxia; FoxO3a; Oxidative stress; Onion; Quercetin; Yuzu; Hesperidin
iii
TABLE OF CONTENTS
ABSTRACT ... i
TABLE OF CONTENTS ... iii
LIST OF FIGURES ... vi
ABBREVIATION ... viii
I. INTRODUCTION ... 1
II. MATERIALS AND METHODS ... 4
A. Transient focal cerebral ischemia ... 4
B. Measurement of brain water content ... 5
C. Measurement of Evans blue extravasation ... 5
D. Immunohistochemistry for TJs ... 5
E. Cell culture ... 6
F. Endothelial cell monolayer permeability assay ... 6
G. Immunocytochemistry for TJs and FoxO3a ... 7
H. Western blot analysis... 7
I. Preparation of cell fractions ... 8
iv
K. siRNA transfection ... 9
L. Measurement of DCF-DA fluorescence... 9
M. Preparation of the OE, Quer, YE, and HSP ... 9
N. Statistical analysis ... 10
III. RESULTS ... 11
A. The effects of OE, Quer, YE, and HSP on water content in brain ischemia model ... 11
B. The effects of OE, Quer, YE, and HSP on Evans blue extravasation in brain ischemia model... 14
C. The effects of OE, Quer, YE, and HSP on TJs disruption in brain ischemia model ... 16
D. The effects of OE, Quer, YE, and HSP on BBB permeability in hypoxia model ... 18
E. The effects of OE, Quer, YE, and HSP on disruption of ZO-1 and claudin-5 in hypoxia model... 22
F. The effects of OE, Quer, YE, and HSP on the expression of MMP-3 and MMP-9 in hypoxia model ... 26
G. The effects of OE, Quer, YE, and HSP on translocation of FoxO3a into nucleus in hypoxia model ... 28
v
I. FoxO3a affects the degradation of claudin-5 but redistribution of ZO-1 in hypoxia
model... 32
J. FoxO3a regulates hypoxia-induced BBB hyperpermeability... 34
K. The effects of oxidative stress scavengers on hypoxia-induced BBB dysfunction in hypoxia model ... 36
IV. DISCUSSION... 40
V. CONCLUSION ... 46
VI. REFERENCES ... 47
vi
LIST OF FIGURES
Fig. 1. Effects of OE and Quer on ischemia-induced water content ... 12 Fig. 2. Effects of YE and HSP on ischemia-induced water content ... 13 Fig. 3. Effects of OE, Quer, YE, and HSP on ischemia-induced Evans blue extravasation .. 15 Fig. 4. Effects of OE, Quer, YE, and HSP on the ischemia-induced disruption of ZO-1 and claudin-5... 17 Fig. 5. Effects of OE, Quer, YE, and HSP on hypoxia-induced hyperpermeability, TEER .. 19 Fig. 6. Effects of OE, Quer, YE, and HSP on hypoxia-induced hyperpermeability, NaF ... 20 Fig. 7. Effects of OE, Quer, YE, and HSP on hypoxia-induced hyperpermeability, Evans blue ... 21 Fig. 8. The effects of OE, Quer, YE, and HSP on hypoxia-induced disruption of TJs ... 23 Fig. 9. The effects of OE, Quer, YE, and HSP on hypoxia-induced redistribution of ZO-1 .. 24
Fig. 10. The effects of OE, Quer, YE, and HSP on hypoxia-induced degradation of claudin-5
... 25
Fig. 11. The effects of OE, Quer, YE, and HSP on hypoxia-induced an increase of MMP-3/9
... 27
Fig. 12. The effects of OE, Quer, YE, and HSP on hypoxia-induced translocation of FoxO3a
... 29
vii
Fig. 14. FoxO3a silencing suppresses hypoxia-induced degradation of claudin-5 ... 33 Fig. 15. FoxO3a silencing suppresses hypoxia-induced BBB hyperpermeability ... 35 Fig. 16. The effects of OE, Quer, YE, and HSP on hypoxia-induced generation of oxidative stress ... 37 Fig. 17. Inhibition of oxidative stress reduces hypoxia-induced BBB hyperpermeability ... 38 Fig. 18. Inhibition of oxidative stress reduces hypoxia-induced degradation of claudin-5, MMPs mRNA levels, and translocation of FoxO3a ... 39
viii
ABBREVIATION
BBB: Blood-brain barrier
DCF-DA: 2’7’-dichlorofluorescein-diacetate FoxO3a: Forkhead box O 3a
HSP: Hesperidin
MCAO: Middle cerebral artery occlusion MMP: Matrix metalloprotease
NAC: N-acetyl-cysteine OE: Onion extract Quer: Quercetin
PEG: Poly(ethylene glycol) ROS: Reactive oxygen species
TEER: Transendothelial electrical resistance TJs: Tight junction proteins
UEA-1: Ulex europaeus agglutinin-1 YE: Yuzu extract
1
I. INTRODUCTION
The blood-brain barrier (BBB) is a physical and metabolic barrier between the peripheral blood circulation and the central nervous system (Sandoval and Witt, 2008). The proper functioning of the BBB is critical for maintaining brain homeostasis and protecting against many toxic compounds and pathogens (Cardoso et al., 2010; Lee et al., 2011). During cerebral ischemia, BBB disruption is a critical event that induces an influx of water, leading to vasogenic edema and secondary brain damage (Pluta, 2005). Brain edema aggravates the progression of infarct formation and causes herniation and death (Walberer et al., 2008). Several studies report that matrix metalloproteinases (MMPs) is the one of the main causes of BBB dysfunction in brain ischemia, degrading tight junction proteins (TJs) (Rosell and Lo, 2008; Tu et al., 2011; Wang et al., 2011). Especially, among MMPs, MMP-9 is involved in BBB dysfunction after brain ischemia (Yang et al., 2007).
It has been reported that reactive oxygen species (ROS) exert deleterious actions, such as BBB disruption and BBB hyperpermeability during vasogenic edema (Pun et al., 2009). Several studies have demonstrated that inhibition of ROS can attenuate brain edema, BBB disruption, and TJs disruption under various pathologic conditions including ischemia (Heo et al., 2005; Pluta et al., 2009; Pun et al., 2009). The amelioration of ischemia-induced BBB disruption and brain edema has also been demonstrated by using dietary antioxidants such as ginkgo biloba extract and olive leaf extract (Pierre et al., 1999; Mohagheghi et al., 2011). In addition, ROS can also contribute to dysfunction of BBB directly and trigger molecular pathways related to MMPs, plasminogen activators, inflammation and vascular endothelial
2
growth factor (VEGF) and those related to vasogenic edema (Heo et al., 2005). Moreover, scavenging superoxide radicals attenuates increased vascular permeability and edema formation in focal cerebral ischemia by reducing activation of MMP-9 (Yang and Rosenberg, 2011).
Forkhead box O (FoxO) belongs to the O type subfamily of the forkhead transcription factor superfamily that are characterized by a conserved forkhead box DNA-binding domain (Polter et al., 2009). Members of the FoxO transcription factor family are FoxO 1, 3a, 4, and 6 in mammalian species (Xie et al., 2012b). Among them, FoxO3a activation induced by oxidative stress and ischemia caused apoptosis through transcription of Bim and Fas ligand (Fukunaga and Shioda, 2009). FoxO3a also transcriptionally regulates a series of downstream targets in the cell cycle, differentiation, and repair of damaged DNA (Salih and Brunet, 2008; Xie et al., 2012b). Lee and colleagues (Lee et al., 2008) reported that the functional activation of MMP-9 via FoxO3a-mediated MMP-3 activation is involved in regulating endothelial cells survival and vascular integrity. Recent studies also reported that FoxO3a may play an important role during injuries involving cerebral ischemia and oxidative stress (Maiese et al., 2007; Fukunaga and Shioda, 2009).
Onion (Allium cepa L.), one of the most widely consumed vegetables, is an important source of dietary antioxidants (Slimestad et al., 2007). Various studies have shown that onion and its major component, quercetin (Quer), are associated with several health benefits such as protection against cardiovascular diseases (Park et al., 2009), atherosclerosis (Vatsala et al., 1980), and cancer (Bianchini and Vainio, 2001). In addition, onion and Quer have a role in the prevention of brain damage such as neuronal death from transient cerebral ischemia
3
(Shri and Singh Bora, 2008; Hwang et al., 2009).
Yuzu (Citrus junos sieb ex Tanaka) is one of the citrus fruits and is mainly cultivated in Korea, China, and Japan. Yuzu is known to improve the blood circulation and inhibit the platelet aggregation (Yu et al., 2011). Hesperidin (HSP), the component of yuzu, is a member of the flavonoid group of polyphenols (Raza et al., 2011). HSP has a protective effect against ischemia-induced characteristic behavioral, biochemical, and histological alteration as well as brain infarction through its anti-oxidative and anti-inflammatory property (Raza et al., 2011). However, little is known about the effect of onion extract (OE), Quer, yuzu extract (YE), and HSP on BBB dysfunction during brain ischemia/hypoxia.
In the present study, it was investigated whether OE, Quer, YE, and HSP affects brain ischemia/hypoxia-induced BBB dysfunction. The possible underlying mechanisms are also investigated, especially those linked to their antioxidant effects and FoxO3a.
4
II. MATERIALS AND METHODS
A. Transient focal cerebral ischemia
All experiments were performed in accordance with the approved animal protocols and guidelines established by the Ajou University School of Medicine Ethics Review Committee for animal experiments. All experiments were performed using male ICR mice (Daehan Biolink, Eumseong, Korea) housed under a controlled temperature and humidity. ICR mice, weighing 30-35g, were anesthetized with a 1.0 ㎖ i.p. injection of an anesthetic cocktail consisting of Ketamine (100 ㎎/㎏, Yuhan Corporation, Seoul, Republic of Korea) and Xylazine (10 ㎎/㎏, Bayer Korea Ltd., Seoul, Republic of Korea) before surgery. Focal cerebral ischemia was induced by the left middle cerebral artery occlusion (MCAO) using an intraluminal filament technique modified from the original rat model (Ding-Zhou et al., 2002). Through a midline neck incision, the left common and external carotid arteries were isolated and ligatured with a 5-0 silk suture (Ethicon, Somerville, NJ). A microvascular clip was temporarily placed across the internal carotid artery. An arteriotomy was fashioned in the common carotid artery just proximal to the carotid bifurcation. A 6-0 nylon monofilament, blunted at the tip with an open flame, was introduced through this incision into the internal carotid artery and advanced 13 mm distal to the carotid bifurcation for occlusion of the origin of the MCA. The thread was carefully withdrawn 2 h after MCAO. All mice were intravenously treated with saline (Sham), OE (100, 300 or 1000 ㎎/㎏), Quer (3, 10 or 30 ㎎/㎏), YE (30, 100 or 300 ㎎/㎏) and HSP (1, 3 or 10 ㎎/㎏) 30 min before
5
MCAO, respectively.
B. Measurement of brain water content
Briefly, brain was removed and separated into ipsilateral and contralateral hemisphere at 24 h after reperfusion. The samples were weighed, then dried for 48 h at 60°C, and weighed again to obtain the dry weight. The brain water content was calculated as (wet weight-dry weight)/wet weight.
C. Measurement of Evans blue extravasation
Briefly, mice received an intravenous injection of Evans blue dye (4 ml/kg of 2% Evans blue in saline) at 10 min after reperfusion. At 24 h after reperfusion, brain from transcardially perfused mice were removed, weighed, placed in 400 μL of pure formamide (Sigma, St. Louis, MO) and incubated for 72 at 50°C. The optical density of the formamide solution was measured at 620 nm. Data are expressed as μg Evans blue/g tissue.
D. Immunohistochemistry for TJs
Briefly, brains were removed at 24 h after reperfusion. Brain tissues were fixed in 4% PFA for 24 h at 4°C and then transferred to 30% sucrose in PBS for 48 h at 4°C. Tissues were then embedded in Tissue-Tek OCT compound (Sakura Finetek Inc, Torrance, CA), frozen at −70°C for 30 min, and sectioned at 20 μm using a frozen section machine (Meyer Instruments, Huston, TX) at −33°C; the sections were mounted on Poly-D-lysine (PDL)-coated glass slides and then soaked in 3% BSA blocking solution at 25°C. Sections were
6
incubated with anti-ZO-1 antibodies (1:50; Invitrogen, Carlsbad, CA) and anti-claudin-5 antibodies (1:100; Invitrogen, Carlsbad, CA) overnight and then with secondary antibody labeled with Alexa 568 (1:500; Invitrogen, Carlsbad, CA). Finally, sections were incubated with FITC-labeled Ulex europaeus agglutinin-1 (UEA-1) (Sigma, St. Louis, MO). All samples were then observed under a Zeiss LSM 510 confocal microscope (Carl Zeiss, Jena, Germany).
E. Cell culture
The bEnd.3 cells was purchased from the ATCC (Manassas, VA, USA) and grown in DMEM (Gibco-BRL, Grand Island, NY) with 10% FBS. For challenge of hypoxia, confluent bEnd.3 cells were incubated in an anaerobic chamber (Forma Scientific, Marietta, OH) at 37 ◦C under an atmosphere composed of 5% CO2, 10% H2 and 85% N2 in glucose free DMEM
that had been saturated with N2 gas for 30 min. All experiments were pretreated with OE (1,
3, or 10 ㎎/㎖), Quer (0.3, 1, or 3 ng/㎖), YE (1, 3, or 10 ㎎/㎖) or HSP (3, 10, or 30 ㎍/ ㎖) 30 min before hypoxic exposure.
F. Endothelial cell monolayer permeability assay
Briefly, bEnd.3 cells were grown on the inside of gelatin-coated transwell insert (0.4 ㎛, Corning Costar, NY) and exposed to hypoxic condition for 4 h. Next, 165 ㎍/㎖ of Evans blue-0.1% albumin (Sigma, St. Louis, MO) and NaF were added to the upper chamber 1 h before measurement. The intensity of the diffused Evans blue-0.1% albumin and NaF in the lower chamber was measured at 650 nm and 485/535 nm, respectively. Results are expressed
7
as ratios of Evans blue-0.1% albumin and NaF concentration in the lower chamber to the total concentration of Evans blue-0.1% albumin and NaF added to the upper chamber at the start of the experiment, respectively. Transendothelial electrical resistance (TEER) across the membrane was measured with a Millicell ERS-2 Voltohmmeter (Millipore CO., Billerica, MA). The gelatin-coated transwell inserts were placed in 24 well plates containing culture medium and then used to measure background resistance. The resistance measurements of these blank filters were then subtracted from those of filters with cells. The values are shown as Ωⅹ㎠ based on culture inserts.
G. Immunocytochemistry for TJs and FoxO3a
Briefly, the samples were fixed with methanol at 25°C and then soaked in 3% BSA blocking solution at 25°C. Samples were incubated with anti-ZO-1 antibodies (1:400; Invitrogen, Carlsbad, CA), anti-claudin-5 antibodies (1:400; Invitrogen, Carlsbad, CA) or anti-FoxO3a antibodies (1:400; Cell signaling Technology, Inc., Danvers, MA) overnight and then with secondary antibody labeled with Alexa 488 (1:500; Invitrogen, Carlsbad, CA). Finally, samples were incubated with Hoechst 33258. All samples were observed under a Nicon C2 confocal microscope (Nicon, Tokyo, Japan).
H. Western blot analysis
To obtain the whole-cell lysate, the cells were lysed and centrifuged at 14,000 rpm for 15 min and the supernatant was collected. For Western blotting, proteins were separated by SDS-PAGE and reacted with anti-ZO-1 antibodies (1:1000, Invitrogen, Carlsbad, CA) or
8
anti-claudin-5 antibodies (1:1000, Invitrogen, Carlsbad, CA) overnight. All sample detected and analyzed using LAS 4000 mini (Fuji Photo Film, Tokyo, Japan).
I. Preparation of cell fractions
After hypoxia exposure, cells were lysed using different lysis buffers and lysates were extracted using different procedures. Briefly, cells were incubated in lysis buffer A (20 mM Tris-HCl, pH 7.4, 250 mM sucrose, 1 mM EDTA, 0.1 mM NaF, 0.2 mM Na3VO4, 0.5 mM
PMSF, 0.01 mM leupeptin, 0.01 mg/ml aprotinin) for 30 min on ice, and lysates were centrifuged at 200,000 x g in a Beckman Optima TL Ultracentrifuge (Beckman Coulter, Brea, CA) at 4°C for 10 min. Supernatants (cytosol fractions) were then removed and remaining pellets were resuspended with lysis buffer B (lysis buffer A containing 1% triton X-100) and incubated on ice for 1 h. Suspensions were the centrifuged as mentioned above and supernatants (membrane fraction) were removed. Pellets were then resuspended in lysis buffer C (lysis buffer B containing 1% SDS) and incubated on ice for 1 h. Suspensions were centrifuged as above and supernatants (actin cytoskeleton fractions) were removed. Samples of each actin cytoskeleton fraction were diluted with SDS sample buffer and incubated at 100°C for 10 min.
J. Polymerase chain reaction (PCR)
Briefly, total RNA was isolated for RT. After RT reaction, cDNA was amplified using specific primers for MMP-3 (forward, 5 – TTG CCA ACC TGC GTA TCT GT; reverse, 5 – TCC CAA GGA TGC CTA GCT CT) and MMP-9 (forward, 5 - CAG CCA GAC ACT AAA
9
GGC CA; reverse, 5 - CCT CGA AGG TGA AGG GAA AG). All sample detected and analyzed using GelDocTM (Bio-Rad, Hercules, CA).
K. siRNA transfection
Briefly, bEnd.3 cells were transiently transfected in siRNA transfection medium with siFoxO3a using siRNA transfection reagent (Santa Cruz Biotechnology, Inc., Dallas, TX).
L. Measurement of DCF-DA fluorescence
Briefly, changes in ROS levels were measured using 2’7’-dichlorofluorescein diacetate (DCF-DA) dye (10 μM) (Molecular Probes, Eugene, OR), a selective fluorescent indicator for ROS. Briefly, bEnd.3 cells grown on was rinsed with HCSS (HEPES controlled salt solution; 120 mM NaCl, 5 mM KCl, 1.6 mM MgCl2, 2.3 mM CaCl2, 15 mM glucose, 20 mM
HEPES, 10 mM NaOH, pH 7.4) and loaded with DCF-DA dye (10 μM) and Pluronic F-127 (10 μM) for 30 min at 37°C in HCSS before hypoxia exposure. After hypoxia, intracellular ROS release induced by 1% triton-X100 were measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
M. Preparation of the OE, Quer, YE, and HSP
Total OE was obtained as previously described (Lancaster and Kelly, 1983) with slight modification. Briefly, after the outer skins or leaves of fresh onions were carefully removed, 50 g of onion bulb was homogenized in 70% methanol (100 ㎖) and the homogenate was filtered through filter paper. The resulting fractions were lyophilized using a vacuum
10
evaporator (N-2N; Eyela, Tokyo, Japan). Lyophilized onion extract was dissolved in phosphate-buffered saline. The gram was used as the unit of measurement for the amount of onion in this study to refer to the mass of fresh onion. YE was obtained by methods slightly modified from a previously described protocol (Wang et al., 2008). Briefly, a mixture of 5 g (fresh weight) of sample and 25 ml of methanol–DMSO (v/v; 50:10) was homogenized in a falcon tube, and stirred on a magnetic stirrer for 10 min. Then 1 ㎖ of the sample was centrifuged at 10,000g for 15 min at 4°C. The supernatant was collected and filtered through a 0.45 lm membrane filter before HPLC analysis. Quer and HSP was purchased from the Sigma (St. Louis, MO, USA).
N. Statistical analysis
All data are expressed as the means ± SEMs. Statistical comparisons were conducted by Student’s t-test and/or one-way analysis of variance (ANOVA). P values of less than 0.05 were considered significant.
11
III. RESULTS
A. The effects of OE, Quer, YE, and HSP on water content in brain ischemia model
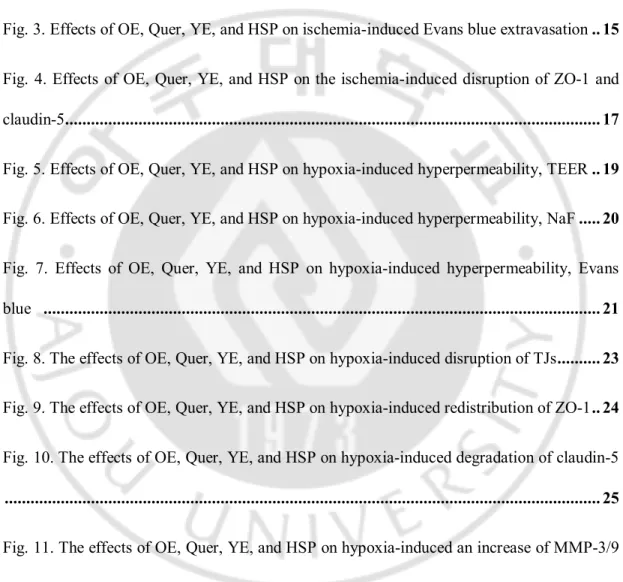
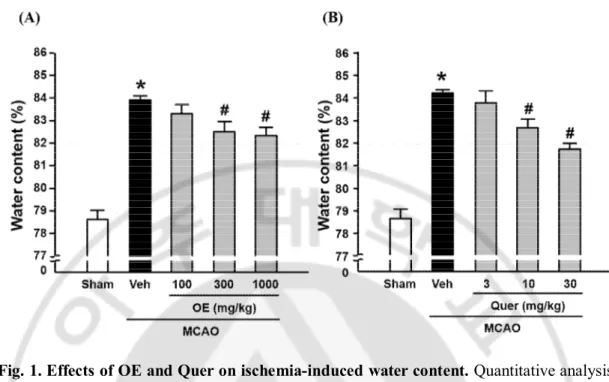
To measure the effects of OE, Quer, YE, and HSP on brain edema in the mouse brain ischemia model, the brain water content was examined using a wet-dry method at 24 h of reperfusion. As shown in Fig. 1A and 1B, brain ischemia caused an increase of cerebral water content in ischemic hemisphere, which was significantly reduced by administration of OE (300 or 1000 ㎎/㎏) or Quer (10 or 30 ㎎/㎏). As shown in Fig. 2A and 2B, brain ischemia caused an increase of cerebral water content in ischemic hemisphere, which was significantly reduced by administration of YE (100 or 300 ㎎/㎏) or HSP (10 ㎎/㎏).
12
Fig. 1. Effects of OE and Quer on ischemia-induced water content. Quantitative analysis
of water contents in brain tissues. Water contents were measured 24 h after MCAO in ischemic hemispheres. All mice were intravenously treated with OE (A; 100, 300, or 1000 ㎎/㎏) or Quer (B; 3, 10, or 30 ㎎/㎏) before MCAO, respectively. All mice were intravenously treated with OE in saline and Quer in 50% of Poly(ethylene glycol) (PEG) 30 min before MCAO. Data shown are mean±SEM. (n=4 or more). #p<0.05 vs. vehicle-treated MCAO (Veh).
13
Fig. 2. Effects of YE and HSP on ischemia-induced water content. Quantitative analysis
of water contents in brain tissues. Water contents were measured 24 h after MCAO in ischemic hemispheres. All mice were intravenously treated with YE (A; 30, 100 or 300 ㎎/ ㎏) and HSP (B; 1, 3 or 10 ㎎/㎏) before MCAO. All mice were intravenously treated with YE in saline and HSP in 50 % of PEG 30 min before MCAO. Data shown are mean±SEM. (n=4 or more). #p<0.05 vs. vehicle-treated MCAO (Veh).
14
B. The effects of OE, Quer, YE, and HSP on Evans blue extravasation in brain ischemia model
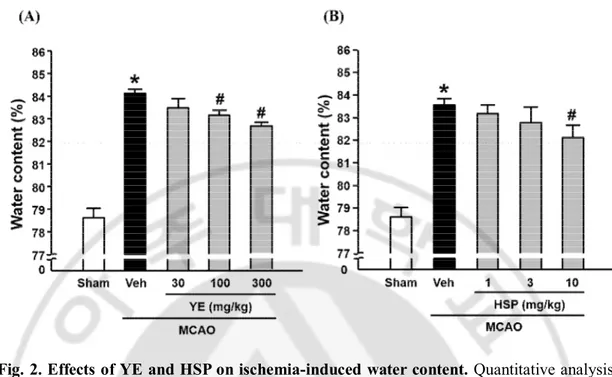
To investigate the effects of OE, Quer, YE, and HSP on BBB dysfunction in the mouse brain ischemia model, it was examined the Evans blue extravasation in ischemic hemisphere at 24 h of reperfusion. As shown in Fig. 3A, brain ischemia caused an increase of Evans blue extravasation in ischemic hemisphere, which was significantly reduced by administration of OE (1000 ㎎/㎏) or Quer (30 ㎎/㎏). As shown in Fig. 3B, brain ischemia caused an increase of Evans blue extravasation in ischemic hemisphere, which was significantly reduced by administration of YE (300 ㎎/㎏) or HSP (10 ㎎/㎏).
15
Fig. 3. Effects of OE, Quer, YE, and HSP on ischemia-induced Evans blue extravasation.
Top, representative photographs of the whole brain. Evans blue extravasation in ischemic hemispheres was observed as dark areas in whole brain. Bottom, quantitative analysis of Evans blue contents in brain tissues. Evans blue contents were measured 24 h after MCAO in ischemic hemispheres. All mice were intravenously treated with OE (A) or YE (B) in saline at 1000 ㎎/㎏ or 300 ㎎/㎏ and Quer (A) or HSP (B) in 50 % of PEG at 30 ㎎/㎏ or 10 ㎎/㎏ 30 min before MCAO, respectively. Data shown are mean±SEM. (n=4 or more). #p<0.05 vs. vehicle-treated MCAO (Veh).
16
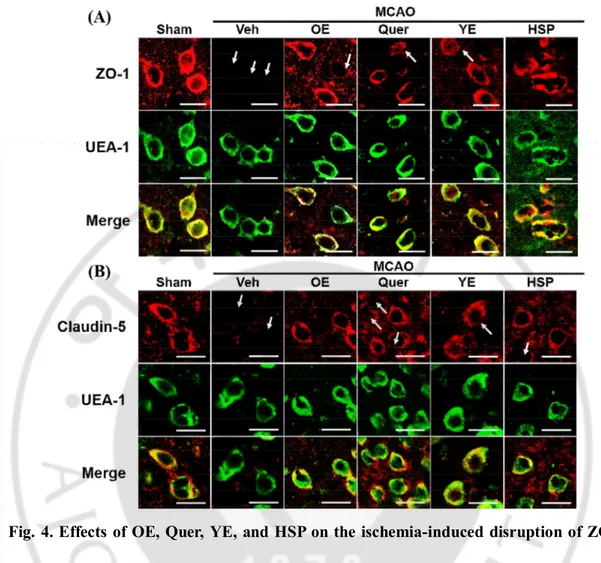
C. The effects of OE, Quer, YE and HSP on TJs disruption in brain ischemia model
To investigate the effects of OE, Quer, YE and HSP on TJs disruption in the mouse brain ischemia model, it was examined ZO-1 or claudin-5 in the periischemic region of mouse MCAO model using immunohistochemistry. As shown in Fig. 4, ZO-1 (A) and claudin-5 (B) staining were normal in sham-operated brain tissues, whereas abnormally discontinuous staining of both was observed around the vessels of ischemic hemispheres, which were stained with UEA-1, an endothelial cell marker. However, this disruption of ZO-1 and claudin-5 was prevented significantly by OE (1000 ㎎/㎏), Quer (30 ㎎/㎏), YE (300 ㎎/ ㎏), or HSP (10 ㎎/㎏) at in the periischemic region of the MCAO group.
17
Fig. 4. Effects of OE, Quer, YE, and HSP on the ischemia-induced disruption of ZO-1 and claudin-5. Representative fluorescence images of ZO-1 (A) and claudin-5 (B).
Immunostaining for ZO-1 and claudin-5 was performed in peri-ischemic regions of ischemic hemispheres at 24 h of reperfusion. All mice were intravenously treated with OE (1000 ㎎/ ㎏), Quer (30 ㎎/㎏), YE (300 ㎎/㎏), or HSP (10 ㎎/㎏) 30 min before MCAO. Endothelial cells were identified using the endothelial marker UEA-1. As shown in merged figure, TJs located on the brain endothelial cells. Arrows indicate regions of TJs disruption. The images are representative of four separate experiments. Scale bar, 20 μm.
18
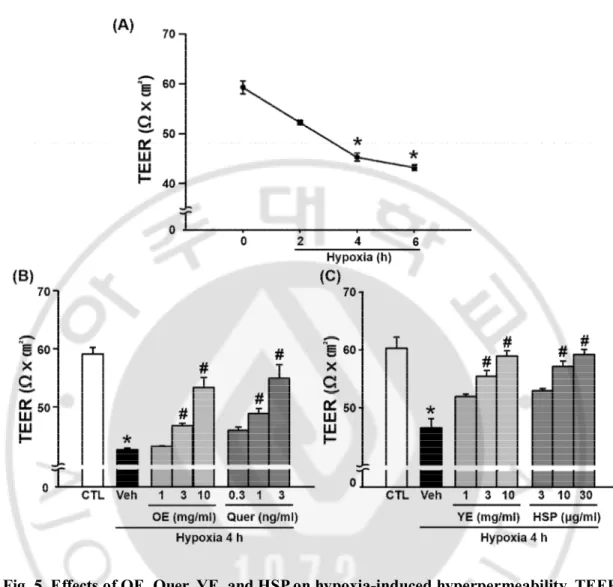
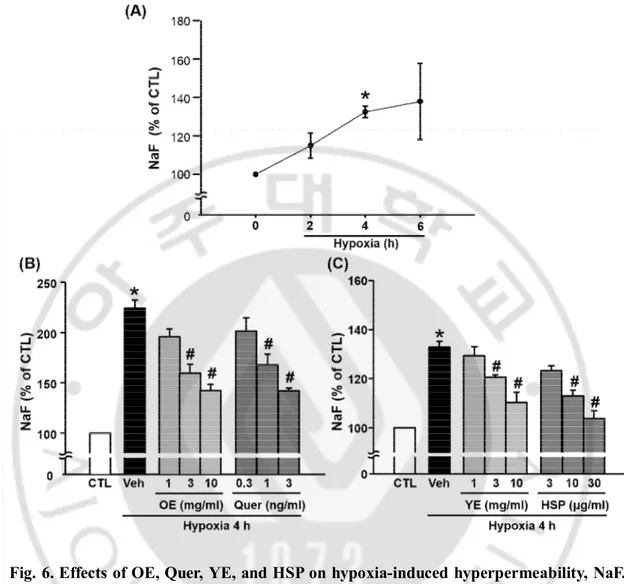
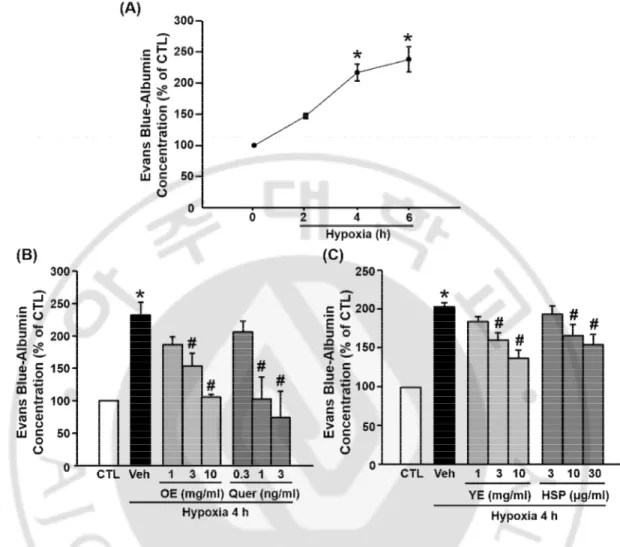
D. The effects of OE, Quer, YE, and HSP on BBB permeability in hypoxia model
To measure the effects of OE, Quer, YE, and HSP on BBB permeability in the hypoxia model, it was examined the BBB permeability using TEER (Fig. 5), NaF (Fig. 6), and Evans blue (Fig. 7). TEER values significantly decreased after 4 h of hypoxia compared with normoxia. The OE, Quer, YE, and HSP prevented hypoxia-induced changes in TEER in a dose-dependent manner. The concentration of Evans blue and NaF in lower chamber was greater in hypoxia group than in the control group, and this increase was significantly attenuated by pre-treatment with OE (1000 ㎎/㎏), Quer (30 ㎎/㎏), YE (3 or 10 ㎎/㎖), or HSP (10 or 30 ㎍/㎏).
19
Fig. 5. Effects of OE, Quer, YE, and HSP on hypoxia-induced hyperpermeability, TEER.
(A) The TEER was measured at control (CTL), 2, 4, and 6 h of hypoxia. (B) Quantitative analysis of TEER in brain endothelial cell monolayer. The TEER was measured 4 h after hypoxia. Data shown are mean±SEM (n=4 or more). *p<0.01 vs. control (CTL), #p<0.01 vs. vehicle-treated hypoxia (Veh). All experiments were pretreated with OE (1, 3, or 10 ㎎/㎏), Quer (0.3, 1, or 3 ng/㎏), YE (1, 3, or 10 ㎎/㎏), or HSP (3, 10, or 30 ㎍/㎏) 30 min before hypoxia.
20
Fig. 6. Effects of OE, Quer, YE, and HSP on hypoxia-induced hyperpermeability, NaF.
(A) The NaF was measured at control (CTL), 2, 4, and 6 h of hypoxia. (B) Quantitative analysis of NaF in transwell. NaF was measured 4 h after hypoxia. Data shown are mean±SEM. (n=4 or more). *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle-treated hypoxia (Veh). All experiments were pretreated with OE (1, 3 or 10 ㎎/㎏), Quer (0.3, 1 or 3 ng/㎏), YE (1, 3 or 10 ㎎/㎏), or HSP (3, 10 or 30 ㎍/㎏) 30 min before hypoxia.
21
Fig. 7. Effects of OE, Quer, YE, and HSP on hypoxia-induced hyperpermeability, Evans blue. (A) The Evans blue was measured at control (CTL), 2, 4, and 6 h of hypoxia. (B)
Quantitative analysis of Evans blue in transwell. Evans blue was measured 4 h after hypoxia. Data shown are mean±SEM. (n=4 or more). *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle-treated hypoxia (Veh). All experiments were prevehicle-treated with OE (1, 3 or 10 ㎎/㎏), Quer (0.3, 1 or 3 ng/㎏), YE (1, 3 or 10 ㎎/㎏), or HSP (3, 10 or 30 ㎍/㎏) 30 min before hypoxia.
22
E. The effects of OE, Quer, YE, and HSP on disruption of ZO-1 and claudin-5 in hypoxia model
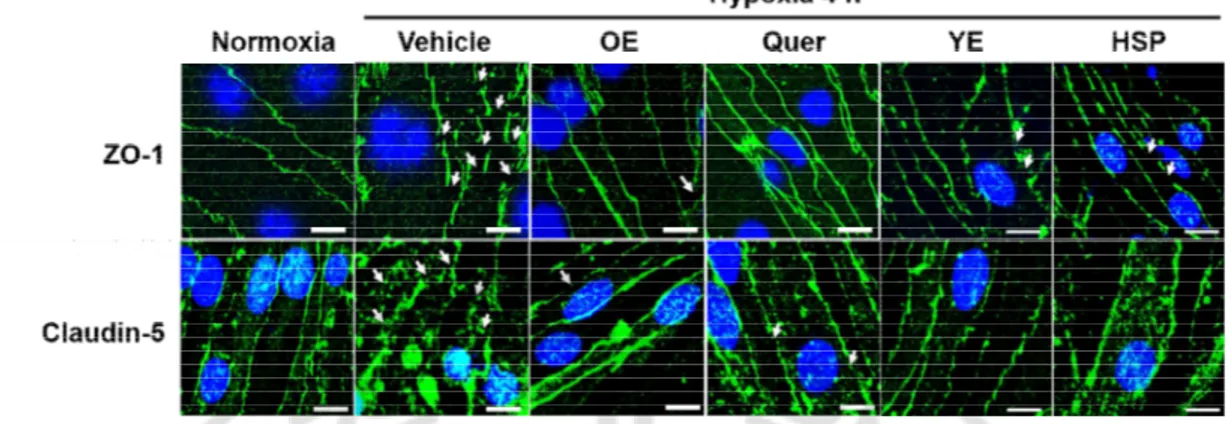
The disruption of ZO-1 and claudin-5 in the hypoxia was quantified using immunocytochemistry and western blotting at 4 h of hypoxia. As shown in Fig. 8, ZO-1 and claudin-5 were disrupted in hypoxia group than in the control group, and this disruption was significantly prevented by pre-treatment with OE (1000 ㎎/㎏), Quer (30 ㎎/㎏), YE (10 ㎎/㎖), or HSP (30 ㎍/㎏).
It has been reported that the disruption of TJs from the plasma membrane can be caused by degradation or translocation of TJs (Liu et al., 2012a; Selvakumar et al., 2013). The consistent with these findings, claudin-5 was degraded at 4 h of hypoxia but ZO-1 was not changed (Fig 10A). Unlike claudin-5, ZO-1 decreased in cytosolic fraction and increased in actin cytoskeletal fraction (Fig 9A), indicating that a translocation of ZO-1 from cytosolic fraction to actin cytoskeletal fraction at 4 h of hypoxia. As shown in Fig. 9B, translocation of ZO-1 was inhibited by pre-treatment with OE (1000 ㎎/㎏), Quer (30 ㎎/㎏), YE (10 ㎎/ ㎖), or HSP (30 ㎍/㎏). As shown in Fig. 10B and 10C, degradation of claudin-5 also was significantly reduced by pre-treatment with OE (1000 ㎎/㎏), Quer (30 ㎎/㎏), YE (10 ㎎/ ㎖), or HSP (30 ㎍/㎏).
23
Fig. 8. The effects of OE, Quer, YE, and HSP on hypoxia-induced disruption of TJs. The
immunofluorescent staining of ZO-1 or claudin-5 (green) and Hoechst 33258 (blue) at 4 h of hypoxia in brain endothelial cells. All experiments were pretreated with OE (10 ㎎/㎏), Quer (3 ng/㎏), YE (10 ㎎/㎏), or HSP (30 ㎍/㎏) 30 min before hypoxia. Arrows indicate disrupted region of ZO-1 and claudin-5. The images are representatives of six separate experiments. Scale bar, 20 μm.
24
Fig. 9. The effects of OE, Quer, YE, and HSP on hypoxia-induced redistribution of ZO-1. Western blotting of ZO-1 and claudin-5. It was detected for ZO-1, claudin-5 at 4 h of
hypoxia. All experiments were pretreated with OE (10 ㎎/㎏), Quer (3 ng/㎏), YE (10 ㎎/ ㎏), or HSP (30 ㎍/㎏) 30 min before hypoxia. CF, cytosolic fraction; MF, membrane fraction; ACF, actin cytoskeleton cell fraction.
25
Fig. 10. The effects of OE, Quer, YE and HSP on hypoxia-induced degradation of claudin-5. Western blotting of ZO-1 and claudin-5. It was detected for ZO-1, claudin-5 at 4
h of hypoxia. All experiments were pretreated with OE (10 ㎎/㎏), Quer (3 ng/㎏), YE (10 ㎎/㎏), or HSP (30 ㎍/㎏) 30 min before hypoxia. Quantitative analysis of Western blots of claudin-5 in (B) and (C). Data shown are mean±SEM. (n=4 or more). *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle-treated hypoxia (Veh).
26
F. The effects of OE, Quer, YE and HSP on the expression of MMP-3 and MMP-9 in hypoxia model
It was investigated whether OE, Quer, YE, and HSP reduced hypoxia-induced increase of MMP-3/9 mRNA levels. The expression of MMP-3 and MMP-9 in the hypoxia was increased at 2 h of hypoxia, but MMP-2 expression did not change throughout the experimental period (A). As shown in Fig. 11B and 11C, the expressions of MMP-3 and MMP-9 were greater in hypoxia group than in the control group, and these increases were significantly prevented by pre-treatment with OE (1000 ㎎/㎏), Quer (30 ㎎/㎏), YE (10 ㎎/㎖), or HSP (30 ㎍/㎏).
27
Fig. 11. The effects of OE, Quer, YE, and HSP on hypoxia-induced an increase of MMP-3/9. (A) The expression of an increase of MMP-3/9 mRNA levels in brain endothelial cells.
The mRNA levels of MMP-3/9 were measured at control (CTL), 1, 2, and 4 h of hypoxia. (B) The expression levels of MMP-3/9 mRNA in brain endothelial cells treated with OE (10 ㎎/㎏), Quer (3 ng/㎏), YE (10 ㎎/㎏), or HSP (30 ㎍/㎏) 30 min before 2 h of hypoxia.
28
G. The effects of OE, Quer, YE and HSP on translocation of FoxO3a into nucleus in hypoxia model
It was investigated whether OE, Quer, YE, and HSP inhibited hypoxia-induced translocation of FoxO3a as the upstream of claudin-5 degradation pathway. The translocation of FoxO3a in hypoxia group was quantified at 1 h of hypoxia (A). As shown in Fig. 12B, the translocation of FoxO3a was greater in hypoxia group than in the control group, and this increase was significantly prevented by pre-treatment with OE (1000 ㎎/㎏), Quer (30 ㎎/ ㎏), YE (10 ㎎/㎖), or HSP (30 ㎍/㎏).
29
Fig. 12. The effects of OE, Quer, YE, and HSP on hypoxia-induced translocation of FoxO3a. The immunofluorescent staining of FoxO3a (green) and Hoechst 33258 (blue) in
brain endothelial cells. (A) The translocation of FoxO3a was measured at control (CTL), 15, 30, and 60 min of hypoxia. Representative images of translocation of FoxO3a at 1 h of hypoxia in (B). All experiments were pretreated with OE (10 ㎎/㎏), Quer (3 ng/㎏), YE (10 ㎎/㎏), or HSP (30 ㎍/㎏) 30 min before hypoxia. Scale bar, 20 μm.
30
H. FoxO3a modulates expression of MMP-3 and MMP-9 in hypoxia model
It was investigated whether FoxO3a regulates hypoxia-induced expression of MMP-3 and MMP-9 by using siFoxO3a in brain endothelial cells. The expression of MMP-3 also was greater in hypoxia group than in the control group, and this increase was significantly prevented by the knockdown of FoxO3a. During hypoxia, however, no differences in mRNA level of MMP-9 were found between vehicle and siFoxO3a groups (Fig. 13A). Western blotting results revealed that protein of FoxO3a in brain endothelial cells was reduced after transiently transfected with 8 ㎍ of FoxO3a siRNA (Fig. 13B).
31
Fig. 13. FoxO3a silencing suppresses hypoxia-induced increase of MMP-3. (A) The
expression levels of MMP-3/9 mRNA in brain endothelial cells. The expression levels of MMP-3/9 mRNA transfected with FoxO3a siRNA (8 ㎍) or control siRNA at 2 h after hypoxia. (B) Brain endothelial cells was transfected with FoxO3a siRNA (siFoxO3a, 4 or 8 ㎍) or control siRNA, as described in Materials and Methods.
32
I. FoxO3a affects the degradation of claudin-5 but redistribution of ZO-1 in hypoxia model
It was investigated whether FoxO3a regulates hypoxia-induced degradation of claudin-5 and redistribution of ZO-1 by using siFoxO3a in brain endothelial cells. As shown in Fig. 14A, the level of claudin-5 was lower in hypoxia group than in the control group, and this decrease was significantly prevented by knocking down FoxO3a. As shown in Fig. 14B, ZO-1 located in cytosolic fraction in normoxia group and The ZO-ZO-1 moved to actin cytoskeletal fraction in hypoxia group. During hypoxia, no significant differences in redistribution of ZO-1 were found between control and siFoxO3a groups.
33
Fig. 14. FoxO3a silencing suppresses hypoxia-induced degradation of claudin-5. (A)
Quantitative analysis of claudin-5 in brain endothelial cells transfected with FoxO3a siRNA or control siRNA at 4 h after hypoxia. Data shown are mean±SEM. (n=4 or more). *p<0.05 vs. normoxia CTL, #p<0.05 vs. hypoxia CTL. (B) Western blotting of redistribution of ZO-1 in brain endothelial cells transfected with FoxO3a siRNA or control siRNA at 4 h after hypoxia.
34
J. FoxO3a regulates hypoxia-induced BBB hyperpermeability
It was investigated whether FoxO3a regulates hypoxia-induced BBB hyperpermeability by using siFoxO3a in brain endothelial cells. The BBB permeability was measured using TEER (Fig. 15A), NaF (Fig. 15B) and Evans blue (Fig. 15C). The inhibition of FoxO3a did not alter TEER values under normoxia, whereas significantly prevented hypoxia-induced changes in TEER. The concentration of Evans blue and NaF in lower chamber was greater in hypoxia group than in the control group, and this increase was significantly attenuated by knocking down FoxO3a with siRNA.
35
Fig. 15. FoxO3a silencing suppresses hypoxia-induced BBB hyperpermeability. (A)
Quantitative analysis of TEER in brain endothelial cell monolayer transfected with FoxO3a siRNA or control siRNA (siCTL) at 4 h after hypoxia. Quantitative analysis of NaF (B) and Evans blue contents (C) in brain endothelial cell monolayer transfected with FoxO3a siRNA or control siRNA at 4 h after hypoxia. Data shown are mean±SEM. (n=4 or more). *p<0.05 vs. normoxia CTL, #p<0.05 vs. hypoxia CTL.
36
K. The effects of oxidative stress scavengers on hypoxia-induced BBB dysfunction in hypoxia model
It has been reported that oxidative stresses are an important factor in BBB dysfunction under ischemic condition (Pun et al., 2009). In this study, therefore, it was confirmed whether oxidative stresses causes BBB dysfunction during hypoxia by using oxidative stresses scavengers, trolox and NAC. First of all, the effects of OE, Quer, YE, and HSP on DCF-DA fluorescence intensity, as an index of oxidative stresses generation, were evaluated after hypoxia. As shown in Fig. 16, the increase in DCF-DA fluorescence intensity after 5 min of hypoxia was decreased by trolox (30 μM) and NAC (30 μM). Next, the relationships of BBB permeability and oxidative stresses after hypoxia were investigated. BBB permeability using TEER (Fig. 17A), NaF (Fig. 17B), and Evans blue-albumin (Fig. 17C) during hypoxia was observed in an in vitro BBB model. The hyperpermeability of BBB during hypoxia was significantly reduced by trolox (30 μM) and NAC (30 μM). As shown in Fig. 18, trolox (30 μM) and NAC (30 μM) also inhibited degradation of claudin-5 (A) during hypoxia. In addition, the increase of MMPs mRNA level (B) and translocation of FoxO3a (C) were attenuated by trolox (30 μM) and NAC (30 μM). These findings suggest that activated oxidative stress may play a deleterious role in the regulation of BBB dysfunction during hypoxia as upstream signaling pathways of FoxO3a-mediated MMPs.
37
Fig. 16. The effects of OE, Quer, YE, and HSP on hypoxia-induced generation of oxidative stress. (A) Quantitative analysis of DCF-DA fluorescence intensity. bEnd.3 cells
were pretreated with DCF-DA for 30 min and then exposed to hypoxia for indicated periods. (B) Fluorescence intensities of DCF-DA were measured at 5 min of hypoxia. Fluorescence intensities of DCF-DA are presented as percentages of fluorescence intensities under normoxic conditions (100%). bEnd.3 cells were pretreated with OE (10 ㎎/㎏), Quer (3 ng/ ㎏), YE (10 ㎎/㎏), HSP (30 ㎍/㎏), trolox (30 μM), or NAC (30 μM) 30 min before hypoxia. Data shown are mean±SEM. (n=4 or more). *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle-treated hypoxia (Veh).
38
Fig. 17. Inhibition of oxidative stress reduces hypoxia-induced BBB hyperpermeability.
(A) Quantitative analysis of TEER in brain endothelial cell monolayer treated with trolox (30 μM) and NAC (30 μM) at 4 h after hypoxia. Quantitative analysis of NaF (B) and Evans blue contents (C) in brain endothelial cell monolayer treated with trolox (30 μM) and NAC (30 μM) at 4 h after hypoxia. Data shown are mean±SEM. (n=4 or more). *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle-treated hypoxia (Veh).
39
Fig. 18. Inhibition of oxidative stress reduces hypoxia-induced degradation of claudin-5, MMPs mRNA levels, and translocation of FoxO3a. (A) Quantitative analysis of
degradation of claudin-5 in brain endothelial at 4 h after hypoxia. Data shown are mean±SEM. (n=4 or more). *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle-treated hypoxia (Veh). (B) The expression levels of MMP-3/9 mRNA in brain endothelial cells at 2 h of hypoxia. (C) The immunofluorescent staining of FoxO3a (green) and Hoechst 33258 (blue) in brain endothelial cells at 1 h of hypoxia. Scale bar, 20 μm. All experiments were pretreated with trolox (30 μM) or NAC (30 μM) 30 min before hypoxia.
40
IV. DISCUSSION
This study demonstrates that OE, Quer, YE, and HSP have protective effects against ischemia/hypoxia-induced BBB dysfunction by inhibiting redistribution of ZO-1 and FoxO3a-mediated degradation of claudin-5.
The BBB is the important system that shields the brain from harmful compounds in the blood (Persidsky et al., 2006). The primary function of the BBB is the strict regulation of permeability (Sandoval and Witt, 2008). It has been reported that the majority of cerebral ischemia-induced brain injuries are related to the disruption of the BBB, which results in edema and secondary damage such as dementia and herniation (Asahi et al., 2001; Pluta, 2005). Indeed, maintenance of the BBB integrity has become an important objective to protect brain from edema (Qu et al., 2009). Recently, it has been reported that the protective effects of natural dietary antioxidants on ischemic brain damage may be attributable to the prevention of BBB disruption (Zhao et al., 2007). In this study, in in vivo model, OE, Quer, YE, and HSP reduced brain edema and BBB hyperpermeability after MCAO, as evaluated by brain water content and Evans blue extravasation. In in vitro model, they also attenuated BBB hyperpermeability after hypoxia, as measured by a value of TEER and a concentration of Evans blue and NaF. The first-line of the defense between blood circulation and brain tissue is the endothelium (Sandoval and Witt, 2008). The endothelium of BBB is strictly limited via physical barrier (paracellular pathway) and transport and metabolic barrier (transcellular pathway) (Persidsky et al., 2006; Abbott et al., 2010). Several studies reported that vasogenic edema is occurred due to paracellular permeability of BBB in cerebral ischemia (Rosenberg and Yang, 2007; Witt et al., 2008; Tu et al., 2011), and thereby it was
41
focused on paracellular permeability of BBB in later experiments. TJs among endothelial cells of BBB form the basic structure of BBB and regulate paracellular permeability (Liu et al., 2012b). TJs are multiprotein complexes that consist of integral transmembrane proteins (occludin and claudins) and cytoplasmic accessory proteins (ZO), and are necessary to maintain BBB integrity (Hyun et al., 2013). Claudins are principal barrier-forming proteins and consist of at least 24 members, with each showing a specific organ and tissue distribution (Niessen, 2007). Especially, brain endothelial cells mainly possess the claudin-5 and possibly some other claudins (Stamatovic et al., 2008). Importantly, claudin-5 deletion resulted in a size-selective increase in permeability in endothelial cells (Stamatovic et al., 2008). The claudin-5-deficient mice also present an altered BBB with higher permeability (Luissint et al., 2012). ZO-1 molecules are located at the cytoplasmic side of the brain microvascular endothelial cell plasma membranes connecting transmembranous TJs such as claudin-5 with the actin cytoskeleton (Niessen, 2007). ZO-1 also serves as a recognition protein for TJs placement and as a support structure for signal transduction proteins (Huber et al., 2001). In the recent study, in addition, hyperpermeability and disruption of BBB are related to ZO-1 during hypoxia (Fischer et al., 2005). In this study, TJs in the ischemic hemisphere of the mouse brain was especially disrupted after MCAO, as demonstrated by the lack of immunoreactivity for ZO-1 and claudin-5 within vessels stained with UEA-1, and that the loss of immunoreactivity was significantly prevented by treatment with OE, Quer, YE, and HSP. The four materials also reduced hypoxia-induced disruption of ZO-1 and claudin-5 when detected immunocytochemistry. Thus, the preventive effect of OE, Quer, YE, and HSP against hyperpermeability of BBB is largely due to its ameliorating effect on
42
structural disruption of TJs during hypoxia.
It is reported that the hypoxia-induced disruption of TJs can be caused by degradation or redistribution of TJs in brain endothelial cells (Kim et al., 2010). Cerebral ischemia induced two parallel processes, degradation of occludin and redistribution of claudin-5 (Liu et al., 2012a). In addition, BBB integrity is paralleled by redistribution and degradation of TJs such as claudin-5 and occludin (Selvakumar et al., 2013).Therefore, it was hypothesized that TJs are redistributed and degraded in parallel during hypoxia. Present study showed that ZO-1 was redistributed in parallel with degradation of claudin-5 at 4 h of hypoxia, consistent with this hypothesis. In addition, OE, Quer, YE, and HSP inhibited the redistribution of ZO-1 and degradation of claudin-5 induced by hypoxia.
MMPs mediate brain ischemia-induced vasogenic edema and BBB dysfunction by degrading the basal lamina proteins, extracellular matrix, and TJs around the BBB (Rosell and Lo, 2008). MMPs are also the final common pathway for disruption of the BBB (Yang and Rosenberg, 2011). The major inducible MMPs are MMP-3 and MMP-9. When these are released, MMP-3 induces an increase of MMP-9 activity, there by result in degradation of TJs such as claudin-5 (Yang and Rosenberg, 2011). Among MMPs, MMP-2 was increased in early ischemic stroke stage, whereas induction of MMP-3 and MMP-9 was increased in late phase (Yang and Rosenberg, 2011). During hypoxia, in this study, MMP-2 mRNA level did not change, whereas MMP-3/9 levels were increased, compared with control group. In addition, several studies reported that among MMPs, especially MMP-9 disrupts the BBB by degrading the TJs (Jin et al., 2011; Lee et al., 2011; Tu et al., 2011).
43
dysfunction (Beard et al., 2014). FoxO1 also increased caveolae-mediated transcytosis across the BBB (Wang et al., 2010). In addition, FoxO4 regulated intestinal permeability through TJs, including ZO-1 and claudin-1 (Zhou et al., 2009). However, little is known that FoxO3a protein has a role in regulation of brain endothelial cell permeability. Lee and colleagues reported that the functional activation of MMP-9 via FoxO3a-mediated MMP-3 activation is involved in regulating EC survival and degrading extracellular matrix (Lee et al., 2008). Emerging data indicated that FoxO3a may play an important role during injuries that involve cerebral ischemia and oxidative stresses (Maiese et al., 2007; Fukunaga and Shioda, 2009). In the results from this study, hypoxia-induced degradation of claudin-5 attenuated by transfection of siFosO3a, thereby it was suggested that FoxO3a related to hypoxia-induced degradation of claudin-5, in part. However, transfection of siFoxO3a did not suppress induced redistribution of ZO-1 in brain endothelial cells, indicating that hypoxia-induced redistribution of ZO-1 does not require FoxO3a. In addition, Lee and colleagues reported that enzymatic activities of MMP-9 increased after FoxO3a activation in endothelial cells but their regulation involves indirect mechanism, MMP-3 activation (Lee et al., 2008). The mRNA and protein levels of MMP-9 were not changed by FoxO3a activation (Lee et al., 2008). FoxO3a-induced MMP-3 activation contributes to increased enzymatic activity of MMP-9 (Lee et al., 2008). The consensus binding site for the forkhead transcription factors was not found in the promoter sequences of MMP-9 (Lee et al., 2008). In the present study,
the knocking down FoxO3a significantly prevented hypoxia-induced BBB
hyperpermeability and mRNA level of MMP-3. Therefore, it was suggested that FoxO3a regulates indirectly the activity of MMP-9 via MMP-3, and then increases the BBB
44
permeability during hypoxia.
ROS are significant contributors to BBB damage and vasogenic edema (Heo et al., 2005). It was recently reported that pharmacological inhibition of ROS may preserve the BBB function, which may ultimately reduce the amount of CNS damage associated with several pathologic conditions (Lochhead et al., 2010). In the present study, OE, Quer, YE, and HSP reduced increase in DCF-DA intensity, ROS indicator, caused by hypoxia. Taken together, it was suggested that the protective effects of OE, Quer, YE, and HSP on dysfunction of BBB may be, at least partially, associated with its antioxidant effect under ischemia/hypoxia.
In the neuronal cell death pathway, oxidative stress causes translocation of FoxO3a transcription factor (Xie et al., 2012a). However, it has not been reported that oxidative stress-induced translocation of FoxO3a causes BBB dysfunction though MMPs during hypoxia. In the present study, OE, Quer, YE, and HSP attenuated hypoxia-induced expression of MMPs mRNA and inhibited translocation of FoxO3a into nucleus. Trolox and NAC also reduced translocation of FoxO3a transcription factor and prevented BBB hyperpermeability and claudin-5 degradation during hypoxia in this study. Indeed, it was reported that oxidative stress is an upstream mediator of ZO-1 redistribution during hypoxia in brain endothelial cells (Park, 2011). In response to oxidative stress, FoxO transcription factors are regulated by several post-transcriptional modifications, including phosphorylation, acetylation, ubiqitination, and methylation (Xie et al., 2012a; Xie et al., 2012b). Among them, phosphorylation of FoxO3a at Thr32, Ser253, and Ser315 increases the association with 14-3-3 protein, which results in translocation of FoxO3a into nucleus (Xie et al., 2012a). Recent
45
study reported that hesperidin inhibited phosphorylation of FoxO3a possibly by increasing Akt pathway (Rong et al., 2013). Therefore, it was hypothesized that OE, Quer, YE, and HSP may suppress translocation of FoxO3a through inhibiting phosphorylation of FoxO3a induced by oxidative stress.
This study is the first to show that OE, Quer, YE, and HSP attenuates ischemia/hypoxia-induced BBB hyperpermeability. This BBB protection involves inhibition of TJs disruption including redistribution of ZO-1 and FoxO3a-mediated degradation of claudin-5. Oxidative stress may play an important role in the regulation of TJs integrity during hypoxia as upstream signaling pathways of redistribution of ZO-1 and FoxO3a-mediated degradation of claudin-5.
46
V. CONCLUSION
In recent years, there has been growing interest in natural substances and their presumed role in the prevention of various diseases (Magalhaes et al., 2009; Park et al., 2009). In addition, natural substances may have fewer side effects than do pharmaceuticals (El-Shemy et al., 2007) and are easily available without prescriptions. In this study, moreover, OE, Quer, YE, and HSP have more protective effects against hypoxia-induced BBB hyperpermeability than antioxidants. In conclusion, the results from this study suggest that OE, Quer, YE, and HSP may be beneficial nutrients for the prevention of BBB function during brain ischemia/hypoxia probably through their antioxidant effects.
47
VI. REFERENCES
1. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ: Structure and function of the blood-brain barrier. Neurobiol Dis 37: 13-25, 2010
2. Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH: Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21: 7724-7732, 2001
3. Beard RS, Jr., Haines RJ, Wu KY, Reynolds JJ, Davis SM, Elliott JE, Malinin NL, Chatterjee V, Cha BJ, Wu MH, Yuan SY: Non-muscle Mlck is required for beta-catenin- and FoxO1-dependent downregulation of Cldn5 in IL-1beta-mediated barrier dysfunction in brain endothelial cells. J Cell Sci 127: 1840-1853, 2014 4. Bianchini F, Vainio H: Allium vegetables and organosulfur compounds: do they help
prevent cancer? Environ Health Perspect 109: 893-902, 2001
5. Cardoso FL, Brites D, Brito MA: Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev 64: 328-363, 2010 6. Ding-Zhou L, Marchand-Verrecchia C, Croci N, Plotkine M, Margaill I: L-NAME
reduces infarction, neurological deficit and blood-brain barrier disruption following cerebral ischemia in mice. Eur J Pharmacol 457: 137-146, 2002
7. El-Shemy HA, Aboul-Enein AM, Aboul-Enein KM, Fujita K: Willow leaves' extracts contain anti-tumor agents effective against three cell types. PLoS One 2: e178, 2007
8. Fischer S, Wiesnet M, Renz D, Schaper W: H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol 84: 687-697, 2005
9. Fukunaga K, Shioda N: Pathophysiological relevance of forkhead transcription factors in brain ischemia. Adv Exp Med Biol 665: 130-142, 2009
10. Heo JH, Han SW, Lee SK: Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med 39: 51-70, 2005
11. Huber JD, Egleton RD, Davis TP: Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci 24: 719-725, 2001
48
12. Hwang IK, Lee CH, Yoo KY, Choi JH, Park OK, Lim SS, Kang IJ, Kwon DY, Park J, Yi JS, Bae YS, Won MH: Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. J Med Food 12: 990-995, 2009
13. Hyun SW, Jang M, Park SW, Kim EJ, Jung YS: Onion (Allium cepa) extract attenuates brain edema. Nutrition 29: 244-249, 2013
14. Jin R, Song Z, Yu S, Piazza A, Nanda A, Penninger JM, Granger DN, Li G: Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke 42: 2033-2044, 2011
15. Kim YA, Park SL, Kim MY, Lee SH, Baik EJ, Moon CH, Jung YS: Role of PKCbetaII and PKCdelta in blood-brain barrier permeability during aglycemic hypoxia. Neurosci Lett 468: 254-258, 2010
16. Lancaster JE, Kelly KE: Quantitative analysis of the S-alk(en)yl-L-cysteine sulphoxides in onion (Allium cepa L.). J Sci Food Agric 34: 1229-1235, 1983 17. Lee HY, You HJ, Won JY, Youn SW, Cho HJ, Park KW, Park WY, Seo JS, Park YB,
Walsh K, Oh BH, Kim HS: Forkhead factor, FOXO3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler Thromb Vasc Biol 28: 302-308, 2008
18. Lee JK, Kwak HJ, Piao MS, Jang JW, Kim SH, Kim HS: Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir (Wien) 153: 1321-1329, 2011
19. Liu J, Jin X, Liu KJ, Liu W: Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 32: 3044-3057, 2012a
20. Liu WY, Wang ZB, Zhang LC, Wei X, Li L: Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther 18: 609-615, 2012b
21. Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP: Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab 30: 1625-1636, 2010
22. Luissint AC, Federici C, Guillonneau F, Chretien F, Camoin L, Glacial F, Ganeshamoorthy K, Couraud PO: Guanine nucleotide-binding protein Galphai2: a
49
new partner of claudin-5 that regulates tight junction integrity in human brain endothelial cells. J Cereb Blood Flow Metab 32: 860-873, 2012
23. Magalhaes PJ, Carvalho DO, Cruz JM, Guido LF, Barros AA: Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Nat Prod Commun 4: 591-610, 2009
24. Maiese K, Chong ZZ, Shang YC: "Sly as a FOXO": new paths with Forkhead signaling in the brain. Curr Neurovasc Res 4: 295-302, 2007
25. Mohagheghi F, Bigdeli MR, Rasoulian B, Hashemi P, Pour MR: The neuroprotective effect of olive leaf extract is related to improved blood-brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine 18: 170-175, 2011
26. Niessen CM: Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol 127: 2525-2532, 2007
27. Park S, Kim MY, Lee DH, Lee SH, Baik EJ, Moon CH, Park SW, Ko EY, Oh SR,
Jung YS: Methanolic extract of onion (Allium cepa) attenuates ischemia/hypoxia-induced apoptosis in cardiomyocytes via antioxidant effect. Eur J Nutr 48: 235-242, 2009
28. Park SR: Regulation mechanism of tight junction proteins during ischemia/hypoxia-induced blood-brain barrier dysfunction (Thesis). Suwon, Ajou University, 2011 29. Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD: Blood-brain barrier: structural
components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 1: 223-236, 2006
30. Pierre S, Jamme I, Droy-Lefaix MT, Nouvelot A, Maixent JM: Ginkgo biloba extract (EGb 761) protects Na,K-ATPase activity during cerebral ischemia in mice. Neuroreport 10: 47-51, 1999
31. Pluta R: Pathological opening of the blood-brain barrier to horseradish peroxidase and amyloid precursor protein following ischemia-reperfusion brain injury. Chemotherapy 51: 223-226, 2005
32. Pluta R, Ulamek M, Jablonski M: Alzheimer's mechanisms in ischemic brain degeneration. Anat Rec (Hoboken) 292: 1863-1881, 2009
33. Polter A, Yang S, Zmijewska AA, van Groen T, Paik JH, Depinho RA, Peng SL, Jope RS, Li X: Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biol Psychiatry 65: 150-159, 2009
50
Res 43: 348-364, 2009
35. Qu YZ, Li M, Zhao YL, Zhao ZW, Wei XY, Liu JP, Gao L, Gao GD: Astragaloside IV attenuates cerebral ischemia-reperfusion-induced increase in permeability of the blood-brain barrier in rats. Eur J Pharmacol 606: 137-141, 2009
36. Raza SS, Khan MM, Ahmad A, Ashafaq M, Khuwaja G, Tabassum R, Javed H, Siddiqui MS, Safhi MM, Islam F: Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Res 1420: 93-105, 2011
37. Rong Z, Pan R, Xu Y, Zhang C, Cao Y, Liu D: Hesperidin pretreatment protects hypoxia-ischemic brain injury in neonatal rat. Neuroscience 255: 292-299, 2013 38. Rosell A, Lo EH: Multiphasic roles for matrix metalloproteinases after stroke. Curr
Opin Pharmacol 8: 82-89, 2008
39. Rosenberg GA, Yang Y: Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus 22: E4, 2007
40. Salih DA, Brunet A: FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 20: 126-136, 2008
41. Sandoval KE, Witt KA: Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 32: 200-219, 2008
42. Selvakumar K, Prabha RL, Saranya K, Bavithra S, Krishnamoorthy G, Arunakaran J: Polychlorinated biphenyls impair blood-brain barrier integrity via disruption of tight junction proteins in cerebrum, cerebellum and hippocampus of female Wistar rats: neuropotential role of quercetin. Hum Exp Toxicol 32: 706-720, 2013
43. Shri R, Singh Bora K: Neuroprotective effect of methanolic extracts of Allium cepa on ischemia and reperfusion-induced cerebral injury. Fitoterapia 79: 86-96, 2008 44. Slimestad R, Fossen T, Vagen IM: Onions: a source of unique dietary flavonoids. J
Agric Food Chem 55: 10067-10080, 2007
45. Stamatovic SM, Keep RF, Andjelkovic AV: Brain endothelial cell-cell junctions: how to "open" the blood brain barrier. Curr Neuropharmacol 6: 179-192, 2008
46. Tu XK, Yang WZ, Liang RS, Shi SS, Chen JP, Chen CM, Wang CH, Xie HS, Chen Y, Ouyang LQ: Effect of baicalin on matrix metalloproteinase-9 expression and blood-brain barrier permeability following focal cerebral ischemia in rats. Neurochem Res 36: 2022-2028, 2011
47. Vatsala TM, Singh M, Murugesan RG: Effects of onion in induced atherosclerosis in rabbits: I. Reduction of arterial lesions and lipid levels. Artery 7: 519-530, 1980
51
48. Walberer M, Ritschel N, Nedelmann M, Volk K, Mueller C, Tschernatsch M, Stolz E, Blaes F, Bachmann G, Gerriets T: Aggravation of infarct formation by brain swelling in a large territorial stroke: a target for neuroprotection? J Neurosurg 109: 287-293, 2008
49. Wang P, Xue Y, Shang X, Liu Y: Diphtheria toxin mutant CRM197-mediated transcytosis across blood-brain barrier in vitro. Cell Mol Neurobiol 30: 717-725, 2010
50. Wang Y-C, Chuang Y-C, Hsu H-W: The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem 106: 277-284, 2008
51. Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM: Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 31: 52-57, 2011 52. Witt KA, Mark KS, Sandoval KE, Davis TP: Reoxygenation stress on blood-brain
barrier paracellular permeability and edema in the rat. Microvasc Res 75: 91-96, 2008
53. Xie Q, Chen J, Yuan Z: Post-translational regulation of FOXO. Acta Biochim Biophys Sin (Shanghai) 44: 897-901, 2012a
54. Xie Q, Hao Y, Tao L, Peng S, Rao C, Chen H, You H, Dong MQ, Yuan Z: Lysine methylation of FOXO3 regulates oxidative stress-induced neuronal cell death. EMBO Rep 13: 371-377, 2012b
55. Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA: Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27: 697-709, 2007
56. Yang Y, Rosenberg GA: Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42: 3323-3328, 2011
57. Yu HY, Park SW, Chung IM, Jung YS: Anti-platelet effects of yuzu extract and its component. Food Chem Toxicol 49: 3018-3024, 2011
58. Zhao J, Moore AN, Redell JB, Dash PK: Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci 27: 10240-10248, 2007 59. Zhou W, Cao Q, Peng Y, Zhang QJ, Castrillon DH, DePinho RA, Liu ZP: FoxO4
inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology 137: 1403-1414, 2009
52 -국문요약-