저작자표시-비영리-동일조건변경허락 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 동일조건변경허락. 귀하가 이 저작물을 개작, 변형 또는 가공했을 경우 에는, 이 저작물과 동일한 이용허락조건하에서만 배포할 수 있습니다.
Histological Changes in Photorejuvenation
Induced by 5-Aminolevulinic Acid
Photodynamic Therapy
in a Photoaged Mouse Model
by
Ji-Youn Park
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Histological Changes in Photorejuvenation
Induced by 5-Aminolevulinic Acid
Photodynamic Therapy
in a Photoaged Mouse Model
by
Ji-Youn Park
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of Master of Medicine
Supervised by
You Chan Kim, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Ji-Youn Park is approved.
SUPREVISORY COMMITTEE
You Chan Kim
Seong Hyang Sohn
You-Sun Kim
The Graduate School, Ajou University
December 23rd, 2011
i
- ABSTRACT-
Histological Changes in Photorejuvenation Induced by
5-Aminolevulinic Acid Photodynamic therapy
in a Photoaged Mouse Model
Background: Photoaging is defined as the premature skin aging induced by ultraviolet (UV)
irradiation, which is histologivally characterized by degradation of collagen fiber and accumulation of abnormal elastotic material in the dermis. Photodynamic therapy (PDT) is a noninvasive technique used in the treatment of various skin disorders, and recently it has been shown to be effective for photoaged skins. However, the histological studies of PDT in animal model have rarely been reported.
Objectives: I observed the histological changes in photoaged mouse skin specimens and in
the specimens after photorejuvenation induced by 5-aminolevulinic acid (ALA)-PDT. Furthermore, I investigated transmission electron microscopic (TEM) studies to observe the differences among normal, photoaged, and post-ALA-PDT biopsy specimens.
Materials and Methods: Total 32 of 6-week-old female albino hairless mice (Skh:hr-1)
were divided into six groups: control group (group A), photoaging group (group B), ALA only group (group C), light-emitting diode (LED) only group (group D), 5-ALA PDT group with 20J/cm2 of light dose (group E), and 5-ALA PDT group with 40J/cm2 of light dose
(group F). For group E, it was divided to 3 identical groups; which were performed from 1 session to 3 sessions of ALA-PDT to investigate the differences among the number of total sessions of PDT. Serial skin biopsies were performed from each mouse at day 2, day 7, day
ii
14, and day 21 from each intervention. For group E, the additional skin biopsies were performed at day 28 from each session. Hematoxylin & Eosin staining, Masson-trichrome staining, and Verhoeff’s elastic staining were performed with additional immunohistochemical staining. Furthermore, TEM studies performed.
Results: During photoaging, both epidermal thickness and dermal thickness were increased,
the amount of dermal collagen fiber was decreased, and the elastotic materials were increased. After ALA-PDT application, both epidermal thickness and dermal thickness were decreased, as the range of the control group. The amount of collagen fiber was increased through day 2 to day 21, and the increased elastotic materials during photoaging period were reversed by 5-ALA PDT. With TEM, the collagen fibers were much decreased during photoaging compared with control group. However, during PDT application, the decreased collagen fibers were remarkably increased. During photoaging, much distended dermal fibroblast with much distended endoplasmic reticulum was noted; however, after PDT application, healthy fibroblast with normalized endoplasmic reticulum was observed. There was minimal difference between PDT with light dose of 20J/cm2 and 40J/cm2 or among the
number of sessions of PDT from one to three. Conclusion:
In conclusion, this study provided the histologic evidence of the beneficial effects of ALA-PDT chronologically for photodamaged skin in mice model. ALA-ALA-PDT induces deposition of collagen in the dermis, normalizes the elastotic materials by photoaging, and normalizes the function of fibroblasts itself. Further studies will be necessary to fully determine the adequate parameters for photorejuvenation.
iii
Keywords: Photodynamic therapy, Photorejuvenation, 5-aminolevulinic acid, mice model,
iv
TABLE OF CONTENTS
ABSTARCT ··· i TABLE OF CONTENTS ··· iv LIST OF FIGURES ··· v LIST OF TABLES ··· vi I. INTRODUCTION ··· 1II. MATERIALS AND METHODS ··· 3
A. ANIMALS AND MICE GROUPING ··· 3
B. UV IRRADIATION FOR PHOTOAGING ··· 5
C. PDT FOR PHOTOREJUVENATION ··· 7
D. SERIAL SKIN BIOPSY ··· 7
E. HISTOLOGIC EXAMINATION ··· 7
1. H&E staining··· 7
2. Masson-trichrome staining and Verhoeff’s elastic staining ··· 8
3. Immunohistochemical staining ··· 8
F. TRANSMISSION ELECTRON MICROSCOPY ··· 9
G. STATISTICAL ANALYSIS ··· 10 III. RESULTS ··· 11 III. DISCUSSION ··· 22 III. CONCLUSION ··· 27 REFERENCES ··· 28 국문요약 ··· 32
v
LIST OF FIGURES
Fig. 1. Increased epidermal thickness and dermal thickness after photoaging ··· 11
Fig. 2. Decreased the amount of collagen fiber and increased elastotic materials in the dermis after photoaging ··· 13
Fig. 3. Decreased ERK expression after photoaging ··· 14
Fig. 4. Increased the amount of collagen fiber and decreased elastotic material in the dermis after 5-ALA PDT in photoaged mice skin ··· 16
Fig. 5. Slightly increased ERK expression after 5-ALA PDT in photoaged mice skin 17
Fig. 6. Differences in the amount of collagen fibers among control, photoaging, and 5-ALA PDT group with transmission electron microscopy ··· 19
Fig. 7. Differences of fibroblasts among control, photoaging, and 5-ALA PDT group with transmission electron microscopy. ··· 20
vi
LIST OF TABLES
Table 1. Mice grouping ··· 4
Table 2. UV irradiated dose for each week and the cumulative irradiated dose ··· 6
1
I. INTRODUCTION
Photoaging is defined as the premature skin aging induced by ultraviolet (UV) irradiation. Clinically, photoaged skin differs from naturally aged skin; it is thickened, rough with coarse wrinkles and mottled pigmentation (Rabe et al., 2006). Histologically, the alterations in dermal collagen fiber, solar elastosis, and the deposition of dystrophic elastotic material within the reticular dermis have been suggested to be one of the major changes in photoaged skin (Montagna et al., 1989; Fisher et al., 1997). UV irradiation induces the synthesis of matrix metalloproteinases (MMPs) in human skin in vivo and MMP-1 is known to be the main enzyme that degrades collagen in the skin (Kahari and Saarialho-Kere, 1997). Also, an impaired transforming growth factor (TGF)-β /Smad pathway, caused by UV irradiation, might play a role in the pathology of photoaged skin (Fisher et al., 2000; Quan et al., 2004). TGF-β is the major regulator of extracellular matrix (ECM) synthesis in human skin; it stimulates fibroblast proliferation in the dermis to enhance collagen synthesis (Inagaki et al., 1994). Recently, the mitogen-activated protein (MAP) kinases, three distinct families of extracellular-signal-regulated kinase (ERK), c-Jun amino-terminal kinase (JNK), and p38 kinase, are thought to be one of the upstream signal transduction pathway in regulating collagen synthesis and degradation (Davis et al., 1996; Chen and Davis, 1999), MMP expression (Gum et al., 1997), and cell growth (Xia et al., 1995). However, the ERK pathway primarily mediates cellular responses to growth factors, whereas the JNK and p38 pathways primarily mediate cellular responses to cytokines and physical stress. Previous
2
study demonstrated that ERK activity is reduced, whereas JNK activity is activated in intrinsically aged human skin in vivo versus young skin (Shin et al., 2005).
Photodynamic therapy (PDT) is a noninvasive technique used in the treatment of various skin disorders, especially in precancerous lesion. Also, there is growing interest in reversing the signs of photoaging using PDT, and the clinical studies have reported over years that the PDT has effects on the rejuvenation of photoaged skins (Touma et al., 2004; Lowe and Lowe, 2005; Gold et al., 2006). However, the histological studies of PDT in animal model have rarely been reported (Choi et al., 2010).Furthermore, the adequate parameter and the number of treatment sessions for photorejuvenation are still undetermined.
In the present study, I observed the histological changes in photoaged mouse skin specimens and in the specimens after photorejuvenation induced by 5-aminolevulinic acid (ALA)-PDT. Furthermore, I investigated transmission electron microscopic studies to observe the differences among normal, photoaged, and post-ALA-PDT biopsy specimens.
3
II. MATERIALS AND METHODS
A. ANIMALS AND MICE GROUPING
6-week-old female albino hairless mice (Skh:hr-1) weighed 25–30 g were obtained from Oriental Co., Ltd. and maintained under individually ventilated cage (IVC) system. All experimental protocols were approved by the Committee for Animal Care and Use at Ajou University. Throughout the experimental protocol, the mice were maintained at standard conditions - temperature 24 ± 2℃, relative humidity 50 ± 10%, and 12 h room light ⁄ 12 h dark cycle. Mice were divided into six groups: control group (group A), photoaging group (group B), ALA only group (group C), light-emitting diode (LED) only group (group D), 5-ALA PDT group with 20J/cm2 of light dose (group E), and 5-ALA PDT group with 40J/cm2
of light dose (group F). For group E, it was divided to 3 identical groups; which were performed from 1 session to 3 sessions of 5-ALA PDT to investigate the differences among the number of total sessions of PDT (Table 1).
4 Table 1. Mice grouping
Groups Methods No. of mice
A Healthy control* 4 B Photoaging 4 C 5-ALA only 4 D LED only (20J/cm2) 4 E 5-ALA PDT (20 J/cm2) 12 † E1 5-ALA PDT (20 J/cm2) x 1 session 4 E2 5-ALA PDT (20 J/cm2) x 2 sessions 4 E3 5-ALA PDT (20 J/cm2) x 3 sessions 4 F 5-ALA PDT (40 J/cm2) 4
5-ALA, 5-aminolevulinic acid; LED, light-emitting diode; PDT, photodynamic therapy * Neither photoaging nor PDT
† There were 3 identical groups; which were performed from 1 session to 3 sessions of ALA PDT to
5 B. UV IRRADIATION FOR PHOTOAGING
For group B to F, UV irradiation was obtained using TL 20W/12 RS UV lamps (Philips, Eindhoven, The Netherlands, range 275-380 nm, peak 310-315 nm). A Kodacel filter (TA401/407; Kodak, Rochester, NY) was mounted 2 cm in front of the UV lamps to remove UVC wavelengths < 290 nm. The UV-dose was measured in minimal erythema dose (MED) using a UV meter (Model 585100; Waldmann Co., Villingen-Schwenningen, Germany). MED is defined as the minimum amount of radiation exposure required to produce erythema with sharp margins after 24h and MED was 100 mJ/cm2. During 5 weeks, the UV irradiation
was administrated from the initial dose of 1.3 MED (130 mJ/cm2) to 3 MED (300 mJ/cm2)
three times weekly. The UV dose was increased weekly by 0.5 to 1 MED up to 3 MED, listed on Table 2. The administrated distance from dorsal skin surface of mice was approximately 17.5 cm.
6
Table 2. UV irradiated dose for each week and the cumulative irradiated dose
Week Sessions Dose (mJ/cm2)
1 1 130 2 130 3 130 2 4 150 5 180 6 180 3 7 225 8 225 9 225 4 10 270 11 270 12 270 5 13 300 14 300 15 300
7 C. PDT FOR PHOTOREJUVENATION
I used 20% 5-ALA (Levulan®, Dusa Pharmaceuticals, Wilmington, MA) as photosensitizer. The solution was applied on the dorsal skin of the mice and the animals were kept in a dark room for 4 hour and the remaining solution was wiped by ethanol and normal saline. The photosensitizer was applied on group C, E, and F.
The LED light irradiation was performed on group D, E, and F, using an incoherent light source (Omnilux reviveTM, 633nm, Phototherapeutics Ltd., Montgomeryville, PA, USA).
D. SERIAL SKIN BIOPSY
After 3 days of resting from the irradiation, all mice got inhalation anesthesia, and serial skin biopsies were performed from each mouse at day 2, day 7, day 14, and day 21 from each intervention. For group E, the additional skin biopsies were performed at day 28 from each session.
E. HISTOLOGIC EXAMINATION
Hematoxylin & eosin staining (H&E), Masson-trichrome staining, and Verhoeff’s elastic staining were performed. Also immunohistochemical staining was performed.
1. H&E staining
Skin biopsies were fixed in 10% formalin, embedded in paraffin, and sectioned into 5-µm sections for routine H&E staining. The epidermal thickness, dermal thickness, and total dermal area were measured. The epidermal thickness was the mean length between the outer sides of the epidermis, excluding the stratum corneum, and the dermo-epidermal
8
junction through the entire section. The dermal thickness was defined as the mean length between the dermo-epidermal junction and upper margin of subcutaneous fat layer. Total dermal area was calculated by the total area from the dermo-epidermal junction to upper margin of subcutaneous fat layer, excluding adnexal structure. Computer-based software (Image-Pro Plus, Media Cyberneticsa, Silver Spring, MD, USA) was used for each measurement. All slides were evaluated under constant magnification (x200) by two observers.
2. Masson-trichrome staining and Verhoeff’s elastic staining
Masson-trichrome (with light green rather than aniline blue) stained collagen fibers and Verhoeff’s stained elastic fibers were quantified using software as mentioned above. For the scanning view of the papillary and upper reticular dermis, constant magnification (x200) was used. In Masson-trichrome stained slides, the amount of dermal collagen fiber was calculated as the ratio of stained area to measured area (SA/MA), presented as the percent expression. The degree of solar elastosis was evaluated as the amount of dystronic or fragmented elastic fiber in the Verhoeff’s stained slides under constant magnification (x400).
3. Immunohistochemical staining
Using the immunoperoxidase technique, 5-µm sections from formalin-fixed, embedded skin samples were stained with antibodies as follows: Rabbit monoclonal phospho-p44/42 MAPK (Erk1/2) antibody (diluted 1:100, Cell Signaling Technology, Beverly, MA, USA), rabbit polyclonal phospho-SAPL/JNK antibody (diluted 1:100, Cell Signaling Technology), rabbit monoclonal phospho-p38 MAPK antibody (diluted 1:50, Cell Signaling Technology),
9
rabbit polyclonal MMP-1 antibody (diluted 1:100, Abnova, Walnut, CA, USA), rabbit monoclonal MMP-3 antibody (diluted 1:100, Epitomics, Burlingame, CA, USA), and rabbit monoclonal TGF-β1 antibody (diluted 1:100, Bioworld Technology, Newmarket Suffolk, UK).
For quantitative evaluation of the immunoreactivity of ERK, JNK, p38, MMP-1, MMP-3, and TGF- β1, the previously mentioned software was used for the immunohistochemically stained sections in the same way it was used for collagen and elastic fiber measurements as percent expression.
F. TRANSMISSION ELECTRON MICROSCOPY
For transmission electron microscopic evaluation, mice skin specimens from each group were used. The specimens were fixed with Karnovsky’s fixative solution (1% paraformaldehyde, 2% glutaraldehyde, 2 mM calcium chloride, 100 mM cacodylate buffer, pH 7.4) for 2 h and washed with cacodylate buffer. After post-fixing with the fixative solution containing 1% osmium tetroxide and 1.5% potassium ferrocyanide for 1 h, tissues were stained with en bloc in 0.5% uranyl acetate, embedded in Poly/Bed 812 resin (Pelco, Redding, CA, USA). The tissues were sectioned by Reichert Jung Ultracut S (Leica, Wetzetlar, Germany) and stained with uranyl acetate and lead citrate. These specimens were observed and photographed under transmission electron microscope (Zeiss, Leo, Oberkochen, Germany).
10 G. STATISTICAL ANALYSIS
A Student’s t test and one-way ANOVA test were used to analyze the statistically significant differences among the staining patterns of biopsy specimen using the SPSS 11.0 statistics program (SPSS, Inc., Chicago, IL, USA). One-way ANOVA was initially performed to determine whether an overall statistically significant change existed before using the two-tailed Student’s t test. A value of p < 0.05 was considered statistically significant. Summary data are expressed as the mean ± standard deviation (SD).
11
III. RESULTS
A. Histologic changes of photoaging 1. Routine histology (H&E staining)
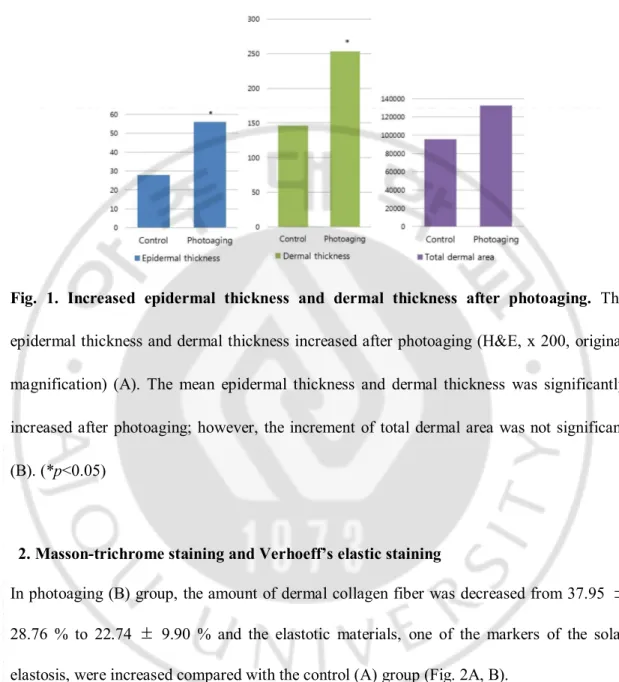
In photoaging (B) group, both epidermal thickness and dermal thickness were increased comparing with the control (A) group (epidermal thickness; from 27.83 ± 1.93 μm to 54.89 ± 4.35 μm after photoaging, dermal thickness; from 145.76 ± 8.84 μm to 252.94 ± 19.15 μm after photoaging) (Fig. 1A, B) (p<0.05). Total dermal area was increased in photoaging group (from 9.55 x 104 ± 0.90 x 104 μm2 to 1.33 x 104 ± 2.85 x 104 μm2 after photoaging);
however, it was not statistically significant (Fig. 1B) (p=0.098).
12 B.
Fig. 1. Increased epidermal thickness and dermal thickness after photoaging. The
epidermal thickness and dermal thickness increased after photoaging (H&E, x 200, original magnification) (A). The mean epidermal thickness and dermal thickness was significantly increased after photoaging; however, the increment of total dermal area was not significant (B). (*p<0.05)
2. Masson-trichrome staining and Verhoeff’s elastic staining
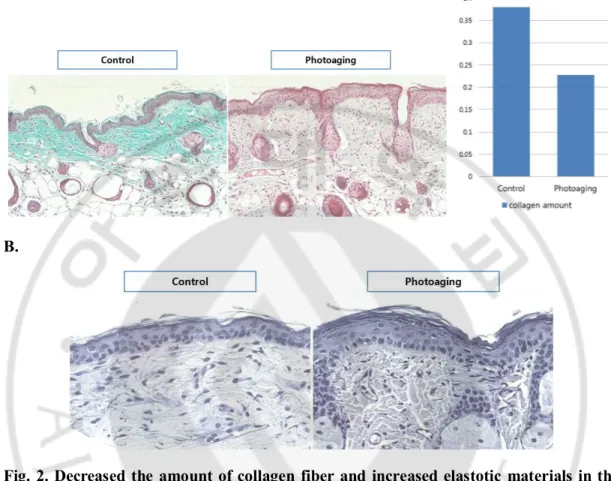
In photoaging (B) group, the amount of dermal collagen fiber was decreased from 37.95 ± 28.76 % to 22.74 ± 9.90 % and the elastotic materials, one of the markers of the solar elastosis, were increased compared with the control (A) group (Fig. 2A, B).
13 A.
B.
Fig. 2. Decreased the amount of collagen fiber and increased elastotic materials in the dermis after photoaging. The amount of collagen fiber was decreased after photoaging
(Masson-trichrome staining, x200, original magnification). (A). The elastotic materials were increased after photoaging, which is one of the indicator of solar elastosis (Verhoeff’s elastic staining, x400, original magnification) (B).
3. Immunohistochemical staining (1) Phospho-p44/42(Erk1/2)
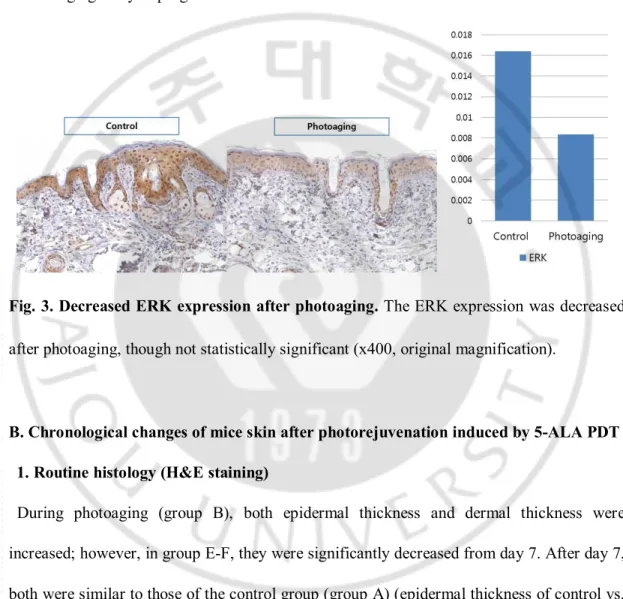
Immunoreactivity of ERK was decreased from 1.63 ± 0.42 % to 0.84 ± 0.76 % in photoaging (group B), compared with control (group A), though not statistically significant
14
(Fig. 3).
(2) Phospho-SAPL/JNK, phospho-p38, TGF-β1, MMP-1, and MMP-3
The staining with above antibodies was minimal; therefore it was inappropriate to analysis with imaging analysis program.
Fig. 3. Decreased ERK expression after photoaging. The ERK expression was decreased
after photoaging, though not statistically significant (x400, original magnification).
B. Chronological changes of mice skin after photorejuvenation induced by 5-ALA PDT 1. Routine histology (H&E staining)
During photoaging (group B), both epidermal thickness and dermal thickness were increased; however, in group E-F, they were significantly decreased from day 7. After day 7, both were similar to those of the control group (group A) (epidermal thickness of control vs. photoaging vs. day 7 group; 27.83 ± 1.93 μm vs. 54.89 ± 4.35 μm vs. 32.83 ± 2.46 μm, dermal thickness of control vs. photoaging vs. day 7 group; 145.76 ± 8.84 μm vs. 252.94 ± 19.15 μm vs. 192.68 ± 17.69 μm). However, there was no consistent change chronologically. Furthermore, total dermal area showed no significant difference during the observed time
15
period.
2. Masson-trichrome staining and Verhoeff’s elastic staining
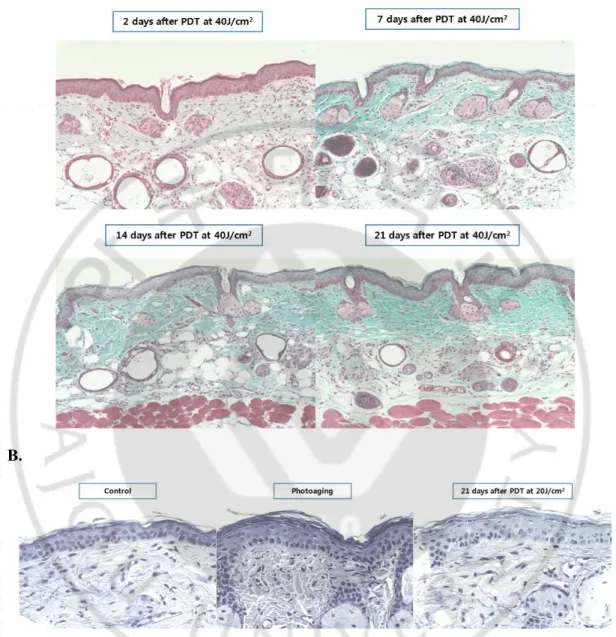
After PDT, the amount of collagen fiber was increased through day 2 to day 21, especially, on day 21 after PDT with 40J/cm2 compared with photoaging group (photoaging vs. day 21
after PDT; 22.74 ± 9.90 % vs. 50.47 ± 15.21 %) (p<0.05) (Fig. 4A). Degraded elastic fibers and increased elastotic materials during photoaging are diminished after 5-ALA PDT (Fig. 4B). The increased elastotic materials, which are known to be one of the histologic hallmarks of photoaging, were reversed by 5-ALA PDT, which means that 5-ALA PDT has an effect on photorejuvenation. With LED only group (group D), the amount of collagen fibers in the dermis was slightly increased comparing with photoaging group; however, it was not statistically significant. Among group E1, E2, and E3, intervened with different number of total sessions of PDT, the dermal collagen amount showed minimal differences.
16
A.
B.
Fig. 4. Increased the amount of collagen fiber and decreased elastotic material in the dermis after 5-ALA PDT in photoaged mice skin. The amount of collagen fiber was
increased through day 2 to day 21, especially, on day 21 after PDT with 40J/cm2 compared
with photoaging group (p<0.05) (A). Degraded elastic fibers and increased elastotic materials during photoaging are diminished after 5-ALA PDT (B).
17 3. Immunohistochemical staining
(1) Phospho-p44/42(Erk1/2)
After PDT, the ERK expression seemed to be increased after PDT with 20J/cm2 compared with phogoaging group (Fig. 5); however, it was not significant as well as not consistent result during the observed time period.
(2) Phospho-SAPL/JNK, phospho-p38, TGF-β1, MMP-1, and MMP-3
The staining with above antibodies was minimal; therefore it was inappropriate to analysis with imaging analysis program.
18
Fig.5. Slightly increased ERK expression after 5-ALA PDT in photoaged mice skin. The
ERK expression was increased after 5-ALA PDT in photoaged mice skin, though not statistically significant. Furthermore, there was no consistent change during the observed time period.
C. Transmission electron microscopy: changes in the quantities of collagen fibers and fibroblasts
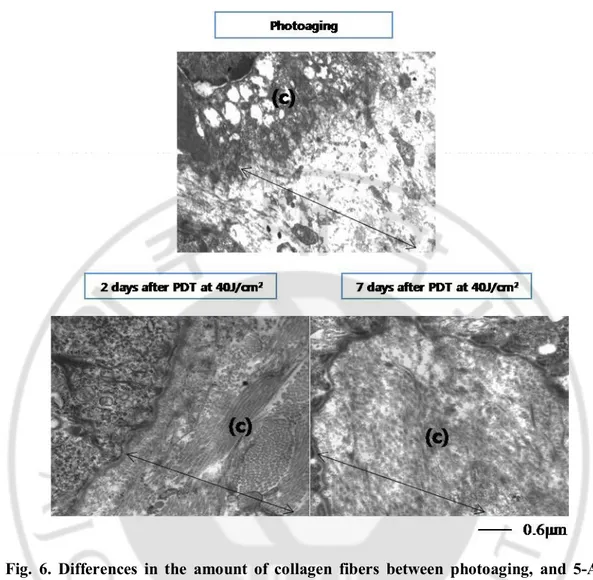
1. Changes in collagen fibers
After photoaging, the collagen fibers (c) were much decreased in the dermis (arrow) compared with control group. However, during 5-ALA PDT application, the decreased collagen fibers were remarkably increased which was filled entire dermis. The increment was noticeable from day 2 after PDT. Furthermore, the amount of collagen fiber from day 2 after PDT exceeded that of control group (Fig. 6).
19
Fig. 6. Differences in the amount of collagen fibers between photoaging, and 5-ALA PDT group with transmission electron microscopy. After photoaging, the collagen fibers
were much decreased compared with control group. However, the decreased collagen fibers were remarkably increased after 5-ALA PDT application. The increment of the amount of collagen fibers was noticeable from day 2 after PDT which exceeded those of control group (arrow: dermis, (c): collagen fibers).
2. Changes in fibroblasts
20
group. During photoaging, much distended dermal fibroblast with much distended endoplasmic reticulum was noted, which means that procollagen synthesized by endoplasmic reticulum cannot be drained freely from fibroblasts. However, after PDT application, healthy fibroblast with normalized endoplasmic reticulum was observed. Also, the electrodense collagen fiber was much more attached with the surface of the fibroblast after PDT application. It seems that the fibroblast is working well with no collagen attenuation in endoplasmic reticulum (Fig. 7). Furthermore, in PDT group, the function of the fibroblast much exceeded that of control group. All above results are summarized on Table 3.
21
Fig. 7. Differences of fibroblasts among control, photoaging, and 5-ALA PDT group with transmission electron microscopy. Normal dermal fibroblasts with normal
endoplasmic reticulum were observed in control group. During photoaging, much distended dermal fibroblast with much distended endoplasmic reticulum was noted. However, after 5-ALA PDT application, healthy fibroblast with normalized endoplasmic reticulum was observed. It seems that the function of fibroblast with no collagen attenuation in endoplasmic reticulum also normalized after PDT application ((f): fibroblast).
22 Table 3. Summary of study results
Control (Group A) Photoaging (Group B) 5-ALA PDT (Group E, F) Histologic changes Epidermal thickness - ↑↑ ↓↓ Dermal thickness - ↑↑ ↓↓
Total dermal area - ↑ ↓
Dermal collagen fiber - ↓↓ ↑↑
Elastotic materials - ↑↑ ↓↓
Immunohistochemical staining
Phospho-p44/42 (Erk1/2) - ↓ ↑
Transmission electron microscopy
Collagen fibers - ↓↓ ↑↑
Fibroblast Normal Distended Normalized
23
IV. DISCUSSION
Photoaging is initiated by cumulative process of environmental damages such as UV irradiation and involves complex alterations of the structural components of the dermal extracellular matrix, especially collagen and elastic fibers (Chung et al., 2001). The histologic hallmarks of photoaging include the loss of collagen and the deposition of elastotic material (Fisher et al., 1997; Lewis et al., 2004). Recently, the increasing interest in reversing photoaging led to emerge the various modalities in dermatologic field, such as chemical peeling and non-ablative and ablative devices.
Topical PDT was originally used for superficial nonmelanoma skin cancers and their precursors. Recently, the efficacy of PDT in other benign diseases, such as acne vulgaris, sebaceous gland hyperplasia, and hidradenitis supprativa, has been introduced (Szeimies et al., 2002). Furthermore, preventing and reversing photoaging by PDT, photorejuvenation, has been widely used (Ruiz-Rodriguez et al., 2002).
The most common photosensitizers used in dermatology are ALA and methyl amonilevulinate (MAL). ALA–PDT utilizes 20% 5-ALA as a prodrug, which, when incorporated in the skin, becomes active in the form of protoporphyrin IX (PpIX) (Gold, 2009). MAL–PDT is the methyl ester of ALA. It also is a prodrug that, when applied to the skin, is converted to PpIX. In contrast to ALA, MAL is more lipophylic, which means it can absorb more deeply into appropriate skin cells (Gold, 2009). Both of them are used in nonmelanoma skin cancer treatment with similar efficacy. There are some reasons why I have chosen ALA as photosensitizer in this study. First, there are much more clinical studies
24
with ALA-PDT in photorejuvenation than those with MAL-PDT. Previous studies demonstrated that ALA-PDT treatment with blue light source had effect on the photorejuvenation and ALA application with intense pulse light (IPL) increased type I collagen production (Gold, 2002; Marmur et al., 2005). Also, the comparative split-face study demonstrated that the 5-ALA-PDT-IPL-treated sides showed greater improvement than the IPL-treated side (Kosaka et al., 2010). On the other hand, the molecular studies and studies with aminal model were little reported so far. The immunohistochemical study with ALA-PDT has been reported by our institute (Park et al., 2010) and the animal model study with MAL-PDT has been introduced recently (Choi et al., 2010). Also, the lack of mice model study of photorejuvenation with ALA-PDT led to initiate this study. Furthermore, the application of photosensitizer on the dorsal skin of mice is much easier with ALA, the liquid form, than MAL, the ointment form.
In this study, I observed the alteration of collagen and elastic fibers among control, photoaging, and post-ALA-PDT in photoaged group. There were numerous clinical observation studies with small sample sizes showing the changes of collagen and elastic fibers after PDT application (Gold, 2002; Marmur et al., 2005; Kosaka et al., 2010). With histological evaluation in animal model, I confirmed the significant decrease in collagen fibers during photoaging (the amount of dermal collagen fiber, from 37.95 ± 28.76 % to 22.74 ± 9.90 %). Also, the decreased collagen fibers by photoaging was reversed with ALA-PDT significantly (the amount of dermal collagen fiber, 45.10 ± 10.56 % at day 20 with ALA-PDT 20J/cm2 and 50.47 ± 15.21 % at day 20 with PDT 40J/cm2). However, they did not showed
25
elastotic materials during photoaging were also reversed with ALA-PDT. However, comparing between PDT with light dose of 20J/cm2 and 40J/cm2, the collagen fibers seemed
not to increase in a dose-dependent manner. Also, with the total two or three sessions of PDT, the amount of collagen fiber showed no significant difference comparing with one session of PDT. It is postulated that one session of PDT might be enough to induce the effect of photorejuvenation. Further studies to demonstrate adequate dose, adequate intervals between sessions, and the number of total sessions of PDT for photorejuvenation will be investigated.
Both epidermal and dermal thicknesses were increased during photoaging, representing the clinical changes of photoaged skins. After UV irradiation, the skin became thickened and rough with coarse wrinkles (Rabe et al., 2006). However, the changes were reversed with 5-ALA-PDT application, which represents that the clinical changes of photoaged skin were also reversed.
To investigate the upstream pathway of producing collagen fiber, immunohistochemical studies with antibodies to MMPs, TGF-β, and MAP kinase were performed. However, the staining with MMPs, TGF-β, JNK, and p38 showed minimal expression not enough to evaluate with imaging analysis. With monoclonal antibodies to ERK, I observed the decreased expression during photoaging. During ALA-PDT, the ERK expression seemed to be slightly increased; however, there was no certain rule of increment depending on the dose intensity, the number of sessions, or variable time period. MAP kinase is one of the signal transduction pathways associated with transcription factors, such as activating protein-1 (AP-1) (Maziere et al., 2003). Families of MAP kinases include ERK, JNK, and p38 which have numerous crosstalks among themselves (Roux and Blenis, 2004). It has been insisted that the
26
balance between the growth factor-activated ERKs and the stress-activated JNKs and p38 pathways influences on the cell survival in the stressful environment (Bickers and Athar, 2006). However, our results were not sufficient to confirm the changes of MAP kinases during photorejuvenation, because the immunohistochemical studies were not enough to investigate the transiently changing signal transduction pathways. Further investigations in molecular levels will be needed. MMPs play a role in the degradation of extracellular matrix components associated with tissue remodeling (Brennan et al., 2003). Fisher et al. (1997) showed that UV irradiation induces MMPs synthesis in human skin in vivo, following collagen degradation (Fisher et al., 1997). During PDT, the decrease in the level of MMPs could play a role in photorejuvenation (Park et al., 2010). However, in this study, the staining with antibodies to TGF-β as well as MMPs showed minimal. It seems that the skin specimens of mice are harder to study with monoclonal antibodies than those of human.
With TEM studies, I have observed the alterations of the collagen fibers and dermal fibroblasts. The degradation of electrodense collagen fibers after photoaging was consistent with the immunohistochemical studies. Furthermore, the reversal of collagen amount after ALA-PDT was also confirmed with TEM. Especially, the increment of collagen fiber was noticeable from day 2 after ALA-PDT, which was prior to the change in immunohistochemical studies. The previous studies showed that the proinflammatory cytokines induced after PDT treatment led to the dermal remodeling, such as degradation of fragmented collagen in early phase (Choi et al., 2010). With TEM, I demonstrated that the collagen fiber started increasing even during early inflammatory phase right after ALA-PDT. The fibroblast normally has extensive rough endoplasmic reticulum which is actively
27
synthesizing protein for export (Breathnach, 1971). When the fibroblast cannot export collagen fiber to the dermis enough, the endoplasmic reticulum would be extended with the procollagen materials (Breathnach, 1971). These changes of fibroblast were observed in photoaged group; however the healthy and well-functioning fibroblast with normalized endoplasmic reticulum reappeared after ALA-PDT application.
There were several previous studies with TEM in photoaged model. Inomata et al. observed the destruction of basement membrane structure and dermal collagen fibers in chronically UV-irradiated mouse (Inomata et al., 2003) and Watson et al. demonstrated the increased dystrophic elastic fibers with sparse microfibrillar apparatus after photoaging and also demonstrated reversely increased deoposition of the microfibrillar dermal matrix components after 4-hr occlusive 0.025% retinoic acid application (Watson et al., 2001). They were focused on the dermal matrix; however, there was no report with the observation of fibroblast after photoaging or phogorejuvenated tools. It is remarkable point that the ALA-PDT could affect normalizing the function of fibroblast itself in this study, which had been more inactive during photoaging, as well as the increment of collagen fibers. It represents that the ALA-PDT application induces not only the transient increment of the amount of collagen fibers but also long-term effect with normalizing function of fibroblast.
28
V. CONCLUSION
In conclusion, this study provided the histologic evidence of the beneficial effects of ALA-PDT for photodamaged skin in mice model. ALA-ALA-PDT induces the deposition of collagen fibers in the dermis and normalizes the dystrophic elastotic materials induced by photoaging. However, the change did not correlate with the course of time, the light dose of PDT, or the total number of sessions of PDT. With TEM, ALA-PDT might have a direct influence on the normalizing function of fibroblast as well as the increment of collagen fibers. Further studies will be necessary to fully determine the adequate parameters of PDT for photorejuvenation.
29
REFERENCES
1. Bickers DR, Athar M: Oxidative stress in the pathogenesis of skin disease. J Invest
Dermatol 126: 2565-2575, 2006
2. Breathnach AS: An atlas of the ultrastructure of human skin: development, differentiation, and post-natal features, Churchill, 1971
3. Brennan M, Bhatti H, Nerusu KC, Bhagavathula N, Kang S, Fisher GJ, Varani J, Voorhees JJ: Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol 78: 43-48, 2003
4. Chen A, Davis BH: UV irradiation activates JNK and increases alphaI(I) collagen gene expression in rat hepatic stellate cells. J Biol Chem 274: 158-164, 1999
5. Choi JY, Park GT, Na EY, Wi HS, Lee SC, Lee JB: Molecular changes following topical photodynamic therapy using methyl aminolaevulinate in mouse skin. J
Dermatol Sci 58: 198-203, 2010
6. Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, Cho KH, Kim KH, Park KC, Eun HC: Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol 117: 1218-1224, 2001
7. Davis BH, Chen A, Beno DW: Raf and mitogen-activated protein kinase regulate stellate cell collagen gene expression. J Biol Chem 271: 11039-11042, 1996
8. Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, Kang S, Voorhees JJ: c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet
30
irradiation is reversed by all-trans retinoic acid. J Clin Invest 106: 663-670, 2000 9. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ: Pathophysiology of
premature skin aging induced by ultraviolet light. N Engl J Med 337: 1419-1428, 1997
10. Gold MH: The evolving role of aminolevulinic acid hydrochloride with photodynamic therapy in photoaging. Cutis 69: 8-13, 2002
11. Gold MH: Therapeutic and aesthetic uses of photodynamic therapy part five of a five-part series: ALA-PDT and MAL-PDT what makes them different. J Clin Aesthet
Dermatol 2: 44-47, 2009
12. Gold MH, Bradshaw VL, Boring MM, Bridges TM, Biron JA: Split-face comparison of photodynamic therapy with 5-aminolevulinic acid and intense pulsed light versus intense pulsed light alone for photodamage. Dermatol Surg 32: 795-803, 2006 13. Gum R, Wang H, Lengyel E, Juarez J, Boyd D: Regulation of 92 kDa type IV
collagenase expression by the jun aminoterminal kinase- and the extracellular signal-regulated kinase-dependent signaling cascades. Oncogene 14: 1481-1493, 1997 14. Inagaki Y, Truter S, Ramirez F: Transforming growth factor-beta stimulates alpha
2(I) collagen gene expression through a cis-acting element that contains an Sp1-binding site. J Biol Chem 269: 14828-14834, 1994
15. Inomata S, Matsunaga Y, Amano S, Takada K, Kobayashi K, Tsunenaga M, Nishiyama T, Kohno Y, Fukuda M: Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol 120: 128-134, 2003
31
16. Kahari VM, Saarialho-Kere U: Matrix metalloproteinases in skin. Exp Dermatol 6: 199-213, 1997
17. Kosaka S, Yasumoto M, Akilov OE, Hasan T, Kawana S: Comparative split-face study of 5-aminolevulinic acid photodynamic therapy with intense pulsed light for photorejuvenation of Asian skin. J Dermatol 37: 1005-1010, 2010
18. Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L: Acquired disorders of elastic tissue: part I. Increased elastic tissue and solar elastotic syndromes. J Am
Acad Dermatol 51: 1-21; quiz 22-24, 2004
19. Lowe NJ, Lowe P: Pilot study to determine the efficacy of ALA-PDT photo-rejuvenation for the treatment of facial ageing. J Cosmet Laser Ther 7: 159-162, 2005
20. Marmur ES, Phelps R, Goldberg DJ: Ultrastructural changes seen after ALA-IPL photorejuvenation: a pilot study. J Cosmet Laser Ther 7: 21-24, 2005
21. Maziere C, Floret S, Santus R, Morliere P, Marcheux V, Maziere JC: Impairment of the EGF signaling pathway by the oxidative stress generated with UVA. Free Radic
Biol Med 34: 629-636, 2003
22. Montagna W, Kirchner S, Carlisle K: Histology of sun-damaged human skin. J Am
Acad Dermatol 21: 907-918, 1989
23. Park MY, Sohn S, Lee ES, Kim YC: Photorejuvenation induced by 5-aminolevulinic acid photodynamic therapy in patients with actinic keratosis: a histologic analysis. J
Am Acad Dermatol 62: 85-95, 2010
32
collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol 165: 741-751, 2004
25. Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN: Photoaging: mechanisms and repair. J Am Acad Dermatol 55: 1-19, 2006
26. Roux PP, Blenis J: ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68: 320-344, 2004
27. Ruiz-Rodriguez R, Sanz-Sanchez T, Cordoba S: Photodynamic photorejuvenation.
Dermatol Surg 28: 742-744, 2002
28. Shin MH, Rhie GE, Kim YK, Park CH, Cho KH, Kim KH, Eun HC, Chung JH: H2O2 accumulation by catalase reduction changes MAP kinase signaling in aged human skin in vivo. J Invest Dermatol 125: 221-229, 2005
29. Szeimies RM, Landthaler M, Karrer S: Non-oncologic indications for ALA-PDT. J
Dermatolog Treat 13 Suppl 1: S13-18, 2002
30. Touma D, Yaar M, Whitehead S, Konnikov N, Gilchrest BA: A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol 140: 33-40, 2004
31. Watson RE, Craven NM, Kang S, Jones CJ, Kielty CM, Griffiths CE: A short-term screening protocol, using fibrillin-1 as a reporter molecule, for photoaging repair agents. J Invest Dermatol 116: 672-678, 2001
32. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270: 1326-1331, 1995
33 -국문 요약-
광노화를
유도한 쥐모델에서 5-ALA 를 이용한
광역동
치료에 의한 광회춘 효과의 조직학적 변화
아주대학교 대학원 의학과 박지윤 (지도교수: 김유찬) 연구 배경: 광노화는 자외선 노출에 의해 발생하는 가속화된 피부 노화로서, 조직학적으로 콜라겐 섬유의 변성과 진피 내의 비정상적인 탄력질양 물질 (elastotic material)의 침착을 특징으로 한다. 광역동 치료는 여러 피부질환에 사용되고 있는 치료법으로서, 최근 광노화된 피부에 효과적이라는 연구들이 있다. 그러나, 동물 모델에서의 조직학적인 변화를 본 보고는 드물었다. 연구 목적: 본 연구에서는 쥐모델을 이용하여 광노화를 시킨 후 조직학적인 변화를 관찰하고, 이 광노화된 쥐모델에 5-aminolevulinic acid (ALA) 을 이용한 광역동 치료을 시행하였을 때 광회춘 효과가 나타나는지 확인하고자 하였다. 또한, 정상 쥐조직, 광노화 조직, 및 광노화 후 광역동 치료를 시행한 쥐조직에서 각각 투과 전자현미경을 이용하여 진피 내 변화가 있는지 관찰하고자 하였다.연구 방법: 총 32 마리의 쥐를 대조군 (A), 광노화군 (B), ALA 처리군 (C), light-emitting diode (LED) 처리군 (D), 20J/cm2 의 ALA-PDT 처리군 (E), 40J/cm2 의 PDT 처리군 (F) 으로 각각 분류하였으며, E 군의 경우 3 군을 세분하여
ALA-34 PDT 를 총 1 회, 2 회, 3 회 시행한 군으로 분류하였다. 연속적으로 피부 조직 검사를 각각의 처리를 시행한 후 2 일, 7 일, 14 일, 21 일에 시행하였으며, E 군의 경우 각 PDT 처리 후 28 일 째에도 추가로 조직 검사를 시행하였다. 각각에 대해 기본 염색과 여러 면역 조직화학 염색 및 투과 전자현미경 연구를 시행하였다. 연구 결과: 광노화 과정 동안, 표피 두께 및 진피 두께는 모두 증가하였고, 진피 collagen 섬유의 양을 감소하였으며, elastotic material 은 증가하였다. ALA-PDT 처리를 시행한 후에는 표피 두께 및 진피 두께는 다시 감소하여 정상과 비슷한 정도로 돌아왔고, 진피 collagen 섬유의 양은 증가, elastotic material 은 감소하여 ALA-PDT 로 인해 광노화된 피부조직이 다시 정상으로 돌아오는 광회춘 효과가 있음을 확인하였다. 투과 전자현미경 상에서, 광노화 시에 감소한 collagen 섬유 역시 ALA-PDT 시행 후 회복되는 모습을 보였고, 광노화 시에 변성되고 기능이 감소한 섬유아세포의 경우에도 ALA-PDT 시행 후 그 기능이 정상화되는 모습 확인할 수 있었다. 결론: 본 연구 결과는 광노화를 유도한 쥐모델에서 ALA-PDT 가 조직학적으로 효과가 있음을 뒷받침하였다. ALA-PDT 로 인해 진피 내 collagen 섬유 증가 및 변성된 elastotic material 이 정상화 되었으며, 비정상적으로 기능이 감소하였던 섬유아세포의 기능도 정상화됨을 확인할 수 있었다. 아울러 추후 적절한 광회춘 효과를 위한 광역동 치료의 parameter 를 결정할 추가적인 연구가 필요하겠다. 핵심어: 광역동 치료, 광회춘 효과, 5-aminolevulinic acid, 쥐 모델, 조직학적 변화, 투과 전자현미경