저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Master’s Thesis in
Medical Sciences
Influencing Factors to Vancomycin
Clearance in Trauma Intensive Care
Unit Patients
Ajou University Graduate School
Medical Sciences Major
Influencing Factors to Vancomycin
Clearance in Trauma Intensive Care
Unit Patients
Young Hwa Choi, M.D., Ph.D., Advisor
I submit this thesis as the
Master
’s thesis in Medical Sciences.
February, 2018
Ajou University Graduate School
Medical Sciences Major
The Master's thesis of Hundo Cho
in Medical Sciences is hereby approved.
Ajou University Graduate School
December 22th, 2017
i ABSTRACT
Influencing Factors to Vancomycin Clearance in Trauma Intensive
Care Unit Patients
Background: Appropriate vancomycin dosing is needed to treat patients with multiple trauma in the
intensive care unit (ICU). The trough level of vancomycin in the trauma intensive care unit (TICU) patients was lower than that of other ICU patients at Ajou University Hospital, a 1,084 bed tertiary hospital. A lower vancomycin concentration may be associated with treatment failure in TICU patients. We evaluated the factors affecting vancomycin levels and studied pharmacokinetics of vancomycin in TICU patients compared to medical ICU (MICU) patients to guide appropriate dosing of vancomycin.
Methods: We retrieved the therapeutic drug monitoring (TDM) database at the Ajou University
Hospital pharmacy retrospectively to select appropriate cases. Patients who were admitted to the TICU or MICU from January 2015 to December 2015 were selected. All patients received at least three doses of vancomycin intravenously and serum vancomycin levels were expected to be steady state. After the expected steady state, the serum vancomycin trough levels and TDM were checked. Patients < 19 years old, those with end stage renal disorder on intermittent hemodialysis or continuous ambulatory peritoneal dialysis and pregnant woman were excluded. Seventy-six cases/56 patients from the TICU and 96 cases/89 patients from the MICU were analyzed. We reviewed patient medical history, laboratory results, use of vancomycin and other drugs, clinical findings, and pharmacokinetics of vancomycin. Continuous variables are expressed as means ± standard deviation, and categorical variables are presented as frequencies and percentages. Group comparisons were analyzed independently by Student’s t-test for continuous variables and the chi-square test for categorical variables. Pearson’s correlation analysis was performed to evaluate the correlations, and the interrelated effects of each variable were analyzed by multiple linear regression. A two-tailed p-value < .05 denoted a significant difference.
Results: Many characteristics of patients were significantly different between the TICU and MICU,
particularly body weight (71.93 ± 14.78, 58.36 ± 19.88; kg; P < .0001) and weight-associated factors, including overweight, overweight rate, body mass index (BMI), body surface area (BSA), and creatinine clearance (143.52 ± 69.41, 97.24 ± 63.69; mg/dl; P < .0001). The pharmacokinetics of vancomycin in the non-continuous renal replacement therapy (CRRT) group revealed the lots of
ii
difference between the two ICUs, and vancomycin clearance was higher in TICU patients than that in the MICU patients (74.25 ± 27.99, 53.74 ± 23.91; ml/min; P < .0001). No significant difference in vancomycin clearance was observed in the CRRT group (35.42 ± 11.03, 30.96 ± 9.64; ml/min; P = .215). The only factor associated with vancomycin clearance in the non-CRRT group was creatinine clearance (coefficient 0.156; P = .017), and no factor was associated with vancomycin clearance in the CRRT group.
Conclusions: Patients with multiple trauma in the ICU were heavier than other ICU patients because
of intensive fluid therapy. A more appropriate dose of vancomycin is required in patients with multiple trauma, depending on the creatinine clearance and actual body weight, and therapeutic vancomycin levels should be monitored. Vancomycin can be infused in fixed doses for patients undergoing CRRT, regardless of the type of ICU or patient characteristics.
_________________________________________________________________________
Key Words: Trauma intensive care unit; vancomycin; therapeutic drug monitoring; creatinine
iii
TABLE OF CONTENTS
ABSTRACT ··· i
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ··· iv LIST OF TABLES ··· v I. INTRODUCTION ··· 1 II. METHODS ··· 2 A. Study Population ··· 2 B. Data Collection ··· 3
C. Vancomycin Concentration Assay ··· 3
D. Statistical Analysis ··· 3
III. RESULTS ··· 5
A. Characteristics of the Study Population ··· 5
B. Pharmacokinetics of Vancomycin ··· 6
C. Factors Associating Vancomycin Clearance ··· 7
IV. DISCUSSION ··· 8
V. CONCLUSION ··· 12
REFERENCES ··· 19
iv
LIST OF FIGURES
v
LIST OF TABLES
▶ Table 1. Clinical characteristics of patients in trauma intensive care unit (TICU) and medical intensive care unit (MICU) without continuous renal replacement therapy (CRRT) ··· 14
▶ Table 2. Pharmacokinetics of vancomycin in trauma intensive care unit (TICU) and medical intensive care unit (MICU) patients without continuous renal replacement therapy (CRRT) and with CRRT ··· 16
▶ Table 3. Linear regression of factors associated with vancomycin clearance in trauma intensive care unit (TICU) patients without continuous renal replacement therapy (CRRT) ··· 17
▶ Table 4. Linear regression of factors associated with vancomycin clearance in patients with continuous renal replacement therapy (CRRT) ··· 18
1
I. INTRODUCTION
Severe trauma causes tissue damage, collapse of the skin barrier, and alterations in the immune system. As a result, severe trauma patients are vulnerable to bacterial invasion and infection. (1, 2) Severe trauma patients can become easily infected with
Staphylococcus aureus, so this should be considered. (3) In particular, methicillin
resistant S. aureus (MRSA) is major pathogen in the intensive care unit (ICU). Vancomycin is the first-line antibiotic for a MRSA infection. (4) Vancomycin is a time-dependent antibiotic, and suboptimal serum concentrations of this drug lead to treatment failure and the development of resistance. (5, 6) Thus, serum vancomycin concentrations must be checked continuously in severe trauma patients, of through using therapeutic drug monitoring (TDM). (7) Ajou University Hospital is a 1,084 bed tertiary hospital with a the trauma intensive care unit (TICU), where suboptimal vancomycin serum concentrations were more frequently observed in severe trauma patients than in other ICU patients.
Vancomycin clearance is influenced by many factors. The patient’s renal function and use of continuous renal replacement therapy (CRRT) have a decisive effect on vancomycin clearance. Capillary leakage of fluid leads to changes in the volume of distribution and vancomycin clearance. (8) Intensive fluid therapy for critically ill patient causes pharmacokinetic alterations due to the hydrophilicity of vancomycin. Simultaneous use of other drugs influencing renal function and use of vasoactive drugs are factors. (5, 9)
Therefore, in this study, we investigated difference in the factors affecting vancomycin levels between patient in the TICU and those the medical intensive care unit (MICU). Furthermore, we statistically analyzed the influence of these factors on vancomycin clearance.
2
II. METHODS
A. Study Population
We retrieved the TDM database at the pharmacy of Ajou University Hospital retrospectively to select appropriative cases. Patients > 18 years, who were admitted to the TICU or MICU from January 2015 to December 2015 were selected. All patients received vancomycin intravenously at least three times, so the serum vancomycin level was expected to be steady state. After the expected steady state, the serum vancomycin levels and TDM were checked. We analyzed the first vancomycin trough level and the TDM, because the clinician adjust the vancomycin dose as result of TDM so the second trough level reach to the target vancomycin level (10– 15 μg/ml). The second TDM and trough level were analyzed as in other cases when a patient received vancomycin and the vancomycin trough level and TDM were checked, since then the patient stopped and restarted vancomycin, if the duration of stop was > 7 days. Exclusion criteria were children < 18 years old, those
with an end stage renal disorder on intermittent hemodialysis or continuous
ambulatory peritoneal dialysis, and pregnant patients. We enrolled 67 patients in the TICU and excluded three patients who did not receive vancomycin more than three times, five patients < 18 years old, and three patients who were undergoing hemodialysis. A total of 108 patients was enrolled from the MICU; 11 patients who did not receive more than three vancomycin doses and eight patients who were undergoing hemodialysis were excluded. The final 76 cases/56 patients from the TICU and 96 cases/89 patients from the MICU were analyzed. This study was approved by the Ajou University Hospital institutional Review Board (AJIRB-MED-MDB-17-161).
3
B. Data collection
We reviewed patients’ medical histories, laboratory results, use of vancomycin and other drugs, as well as clinical findings. Among the patients’ characteristics, weight was recorded specifically as actual body weight, ideal body weight (IBW; kg; male: 50 + 0.91 × (height (centimeter) − 152.4), female: 45.5 + 0.91 × (height (centimeter)-152.4)), overweight (kg; weight – IBW), overweight rate (percent; (overweight/ideal
body weight) × 100), and body mass index (BMI; kg/m2). Fluid input and output
were analyzed specifically as total fluid input, day fluid output per weight, and 1-day urine output per weight. Use of drugs that affected renal function (vasoactive agents, angiotensin receptor blockers, non-steroidal anti-inflammatory drugs, mannitol, aminoglycoside, and furosemide), number of simultaneously used antibiotics, and furosemide dosage were also analyzed. The clinical severity of the patient was evaluated with the Injury Severity Score in the TICU and the Simplified Acute Physiology Score 3 in the MICU. (10)
C. Vancomycin concentration assay
Sampling to measure serum vancomycin trough levels was done 1 hour before the next dose. The samples were analyzed by fluorescence polarimetry (Cobas Integra 800, Roche Diagnostics, Mannheim, Germany).
D. Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables are presented as frequencies and percentages. Group comparisons were analyzed independently by the Student’s t-test for continuous variables and the chi-square test for categorical variables. Pearson’s correlation analysis test was done to evaluate the correlations between the variables, and interrelationships of each
4
variable were analyzed by multiple linear regression. A two-tailed p-value of < .05 denoted a significant difference. All statistical analyses were performed with IBM SPSS Statistics (version 21.0; IBM Corp., Armonk, NY, USA).
5
III. RESULTS
A. Characteristics of the study population
Table 1 shows the clinical characteristics and laboratory findings of the TICU and MICU non-CRRT patients. Fifty-six patients in the TICU were enrolled and 89 patients in the MICU were enrolled. The mean age of the TICU patients (50.17 ± 19.1 years) was significantly lower than that of the MCIU patients (67.41 ± 14.35 years; p < .000). However, height (168.91 ± 8.14, 164.05 ± 8.43; cm; p = .002) and actual body weight (71.93 ± 14.78, 58.36 ± 19.88; kg; p < .000) of the TICU patients were significantly higher than those of the MICU patients. Mean IBW (64.28 ± 8.34, 59.35 ± 9.02; kg; p = .002), overweight (7.65 ± 14.57, 7.65 ± 14.57; kg; p = .002),
BMI (25.20 ± 5.02, 21.49 ± 5.79; kg/m2; p < .000), body surface area (BSA; 1.82 ±
0.20, 1.61 ± 0.25; cm0.725 ×kg0.425 × 0.007184; p < .000) were higher in TICU patients
than those in MICU patients. No significant differences were observed in sex, prevalence of diabetes mellitus, or chronic kidney disease between the two groups. The rate of therapeutic use of vancomycin versus empiric use (60.4%) was significantly higher in TICU patients than that in MICU patients (31.3%; p = .002). The rate of a blood stream infection was higher in TICU patients (27.1%) than that in MCIU patients (6.0%; p = .001). The rate of pneumonia in TICU patients (56.2%) was lower than that in MICU patients (92.7%; p < .000). The rate of soft tissue infection in TICU patients (16.7%) was higher than that in MICU patients (1.2%; p = .001).
Creatinine clearance (CCr; 143.52 ± 69.41, 97.24 ± 63.69; ml/min; p < .000) and total bilirubin (3.29 ± 4.96, 0.96 ± 0.96; mg/dl; p = .002) were significantly higher in TICU patients than those in MICU patients. However, serum blood urea nitrogen,
6
creatinine, albumin, total protein, white blood cell count, platelet count, and pH were not different between the two groups.
Total fluid input from hospitalization in the ICU to checking serum vancomycin concentration was not different between the two groups (2,780 ± 14,382, −214 ± 9,389 ml; p = .200). The 1-day urine output per weight of the TICU patients (60.76 ± 54.83 ml/kg/day) was higher than that of the MICU patients (41.32 ± 17.73 ml/kg/day; p = .021). No difference in 1-day urine output per weight was observed between the two groups (45.98 ± 32.99, 39.63 ± 17.89; ml/kg/day; p = .155).
The rate of use of vasoactive agents by TICU patients (16.7%) was lower than that of MICU patients (42.2%; p= .002), and use of mannitol by TICU patients (8.3%) was higher than that by MICU patients (0.0%; p = .017). The rate of use of furosemide by TICU patients (6.3%) was lower than that by MICU patients (49.3%; p < .000). Total volume (3.54 ± 20.36, 20.24 ± 30.52; mg; p< .000) and 1-day mean volume (1.17 ± 6.77, 7.36 ± 10.32; mg/day; p < .000) of furosemide used by TICU patients during the vancomycin administration period was significantly lower than that of MICU patients. (Table 1)
B. Pharmacokinetics of vancomycin
No significant difference in total vancomycin dose was observed in patients who were not undergoing CRRT between the two ICU groups (5.19 ± 1.81, 4.90 ± 1.60; g; p = .347). The 1-day mean vancomycin dose was not different between the two groups (1.96 ± 0.45, 1.96 ± 0.45; g/day; p = .477). However, 1-day mean vancomycin dose per weight was significantly lower in TICU patients than that in MICU patients (27.85 ± 5.88, 34.71 ± 9.24; mg/kg/day; p < .000). The serum vancomycin peak and trough levels were lower in TICU patients than those in MICU patients (peak level, 25.95 ± 9.95, 32.64 ± 10.79; μg/ml; p = .001; trough level, 10.42 ± 8.13, 14.63 ±
7
8.47; μg/ml; p = .006). The volume of distribution of vancomycin was lower in TICU patients than that in MICU patients (51.12 ± 7.92, 45.82 ± 8.70; L; p = .001). We calculated the optimal vancomycin dose through the initial dosage and serum vancomycin trough level, and the optimal vancomycin dose was higher in TICU patients than that in MICU patients (3,251 ± 1,647, 2,094 ± 1,101; mg, p < .000). Vancomycin clearance was significantly higher in TICU patients than that in MICU patients (74.25 ± 27.99, 53.74 ± 23.91; ml/min; p < .000).
No significant differences in total vancomycin dose, 1-day vancomycin dose per weight, serum vancomycin peak level, serum vancomycin trough level, half-life of vancomycin, optimal vancomycin dose, or vancomycin clearance were observed between the two ICU groups in patients undergoing CRRT (Table 2).
C. Factors associating vancomycin clearance
We evaluated the factors associated with vancomycin clearance in TICU patients who were not undergoing CRRT by linear regression (Table 3). The only factor
associated with vancomycin clearance in the non-CRRT group was serum CCr (R2
= .559; p < .000; coefficient = .156, p = .017). We evaluated the factors influencing vancomycin clearance in all ICU patients who were undergoing CRRT (Table 4). However, no factor was associated with vancomycin clearance, including sex, height, actual body weight, BMI, concurrence chronic kidney disease, total bilirubin, or
8
IV. Discussion
In this study, we compared TICU patients and MICU patients to find factors associated with vancomycin clearance. The patient factors that were significantly different between TICU and MICU patients were age, height, actual body weight, IBW, overweight, BMI, BSA, therapeutic use of vancomycin (vs. empiric use), kind of infection, serum CCr, serum total bilirubin, 1-day fluid output per weight, use of vasoactive agents, use of mannitol, use of furosemide, and dose of furosemide.
Trauma patients develop alterations of the immune system through tissue damage. As a result, capillary leakage and peripheral fluid retention occur. (1) These patients should undergo intensive fluid therapy to stabilize their hemodynamics. These processes affect the pharmacokinetics of drugs, augment renal elimination of drugs, and increase the volume of distribution, and decrease serum drug concentration. (8, 9, 11) Weight gain occurs as a result of traumatic damage and massive fluid therapy, so it reflects the degree of damage and treatment intensity. And we expect that drug clearance increases with weight gain.
In this study, the largest differences between the two ICU patient groups were found in the weight indices. TICU patients were heavier and taller than MICU patients and had a higher IBW and overweight. The overweight rate, which is overweight divided by IBW, was 12.98 ± 24.8% in TICU patients who undergo high intensity therapy and have a high degree of tissue damage. The MICU patients’ IBW was lower than their actual body weight, which may have resulted from the longer hospitalization and long-term management of pulmonary edema in the MICU patients.
In this study, serum CCr was measured using the Cockcroft–Gault formula. This formula includes serum creatinine, age, and actual body weight. Serum CCr was
9
largely different between the TICU and MICU patients (143.52 ± 69.41, 97.24 ± 63.69; ml/min; p < .000), whereas serum creatinine was not different (0.79 ± 0.69, 0.73 ± 0.41; mg/dl; p = .492), so we expected that these differences originated from patient’s age and their actual body weight.
Table 2 shows the pharmacokinetics of vancomycin compared between the two groups according to use of CRRT. The 1-day vancomycin dose in the non-CRRT group was not different between the two ICU patient groups, and the actual body weight was higher in TICU patients, so we expected that the 1-day vancomycin dose per weight would be lower in TICU patients than that in MICU patients. The doctors of both ICU patients did not consider patient factors and used vancomycin at a fixed dose. Actually, vancomycin is generally administered at a rate of 2 g/day to patients in Ajou University Hospital. Heavier TICU patients were administered a relatively lower dose, and vancomycin clearance was higher in TICU patients, so the serum vancomycin peak and trough levels were lower in TICU patients than those in MICU patients. In particular, the serum vancomycin trough level was 10.42 ± 8.13 μg/ml in TICU patients and this value was lower than the optimal vancomycin trough level for pneumonia treatment (15 μg/ml). (7)
Vancomycin clearance in the CRRT group was not different between the two ICU patient groups. CRRT was the only method that eliminated vancomycin, so vancomycin clearance was not affected by the patient characteristics. Thus, vancomycin clearance should not be considered to determine the vancomycin dose according to ICU type.
Table 3 shows the correlations between patient factors and vancomycin clearance in the TICU patients who were not undergoing CRRT through linear regression. The only factor that was significantly correlated was serum CCr. The correlation between
10
vancomycin clearance and serum CCr has been confirmed by studies with non-severe trauma patients and neurosurgical ICU patients, and by our study. (12, 13)
As mentioned above, serum CCr includes patient age, actual body weight, and serum creatinine concentration. In our study, these three factors did not separately affect vancomycin clearance, but they were correlated with vancomycin clearance when they were considered simultaneously. Thus, serum CCr including patient’s actual body weight should be considered when determining vancomycin dose.
Medellin-Garibay et al. reported that use of furosemide in trauma patients affects the pharmacokinetics of vancomycin, but our study showed no significant correlation with use of furosemide. (13) In our study, the severe trauma patients were hospitalized in the TICU, so the subjects were different from those in a previous study. The number of patients using vancomycin was small (n = 3, 6.3%) and the vancomycin dose was too small (1.17 ± 6.77 mg/day). Thus, more studies are needed. Use of CRRT may affect drug clearance. In particular, drugs with high molecular weights, such as vancomycin, are affected more by the intensity of CRRT than by the CRRT method. (14, 15) In our study, many factors were analyzed to determine their association with vancomycin clearance in the CRRT group, but none of the factors were correlated, including the intensity of CRRT (Table 4). The difference between prior studies and our study was that our subjects included trauma patients. TICU patients differ from MICU patients based on the volume of fluid therapy and actual body weight, which affect pharmacokinetics of drugs, and these differences might be the reason for the differences between our study and prior studies.
No difference in vancomycin clearance in the CRRT group was observed between the two ICU patient groups (Table 2), and no factor affected vancomycin clearance in both sets of ICU patients. (Table 4) Therefore, vancomycin dosing can be done using a fixed dose in patients undergoing CRRT.
11
Several limitations of this study should be mentioned. First, the sample size was relatively small to identify differences between the two groups. Second, this study was conducted at a single referral center in Far East Asia, so the results may not be applicable to other regions. Third, the number of patients who used furosemide was too small. Furosemide is an important drug affecting drug clearance. In this study the influence of furosemide could not be detected exactly.
12
V. CONCLUSION
Patients with multiple traumas in the ICU were heavier than other ICU patients because of intensive fluid therapy. Therefore, a more appropriate dose of vancomycin is required in patients with multiple traumas, depending on the CCr and actual body weight. And therapeutic vancomycin levels should be monitored. Vancomycin can be infused at a fixed dose, regardless of ICU type and patient characteristics in patients undergoing CRRT.
13 Figure 1. Study population
TICU, trauma intensive care unit; MICU, medical intensive care unit; CRRT, continuous renal replacement therapy
Inclusion criteria
∙Over three times infusion of vancomycin ∙Therapeutic drug monitoring done
∙Previous infusion of vancomycin above 7days Exclusion criteria
∙Age under 18 years old ∙End stage renal disorder
∙Intermittent hemodialysis
∙Continuous ambulatory peritoneal dialysis ∙Pregnancy Analyzed cases/patients (n=76/56) Analyzed cases/patients (n=96/89) Excluded patients (n=11) Excluded patients (n=19) Trauma patients in TICU
(n=67) Medical patients in MICU (n=108)
Cases/patients with CRRT (n=28/21) Cases/patients without CRRT (n=48/25) Cases/patients without CRRT (n=83/78) Cases/patients with CRRT (n=13/13)
14
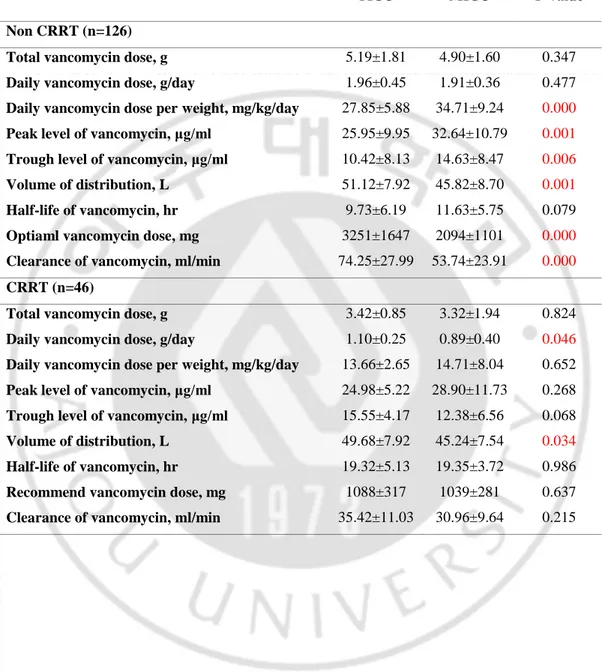
Table 1. Clinical characteristics of patients in trauma intensive care unit (TICU) and medical intensive care unit (MICU) without continuous renal replacement therapy (CRRT)
TICU (n=56) MICU (n=89) P-value Characteristics of patients
Age, years 50.17±19.14 67.41±14.50 0.000
Height, cm 168.91±8.14 164.05±8.43 0.002
Actual body weight, kg 71.93±14.78 58.36±19.88 0.000
Ideal body weight, kg 64.28±8.34 59.35±9.02 0.002
Overweight*, kg 7.65±14.57 -0.20±17.0 0.005 Overweight rate**, % 12.98±24.86 -1.56±25.87 0.002 BMI, kg/m2 25.20±5.02 21.49±5.79 0.000 BSA, cm0.725 x kg0.425 x 0.007184 1.82±0.20 1.61±0.25 0.000 Male, n (%) 40 (83.3) 60 (72.3) 0.201 DM, n (%) 9 (18.7) 24 (28.9) 0.217 CKD, n (%) 0 (0) 2 (2.4) 0.532 Type of disease
Therapeutic use of vancomycin (versus, empirical), n (%)
29 (60.4) 26 (31.3) 0.002 Blood stream infection, n (%) 13 (27.1) 5 (6.0) 0.001
Pneumonia, n (%) 27 (56.2) 77 (92.7) 0.000
Soft tissue infection, n (%) 8 (16.7) 1 (1.2) 0.001
Laboratory finding BUN, mg/dl 19.24±13.38 22.73±13.54 0.157 Cr, mg/dl 0.79±0.69 0.73±0.41 0.492 Ccr***, ml/min 143.52±69.41 97.24±63.69 0.000 Albumin, g/dl 3.21±0.40 3.09±0.56 0.175 Protein, g/dl 5.55±0.93 5.85±1.30 0.174 Total bilirubin, mg/dl 3.29±4.96 0.96±0.96 0.002
White blood cell count, /μl 13154±7063 13055±6360 0.935
Platelet count, ×103/mm3 291±165 247±174 0.161
pH 7.42±0.07 7.43±0.06 0.330
Fluid control
Total fluid intake, ml 2780,
[-4411,9971]
****
-214, [-4480,4908]
15
Daily fluid output per weight, ml/kg/day 60.76±54.83 41.32±17.73 0.021 Daily urine output per weight, ml/kg/day 45.98±32.99 39.63±17.89 0.155
Combined drug
Number of used antibiotics, n 1.40±0.76 1.43±0.66 0.767 Patient using vasoactive agent, n (%) 8 (16.7) 35 (42.2) 0.002
Patient using ARB, n (%) 3 (6.3) 11 (13.2) 0.254
Patient using NSAIDs, n (%) 6 (12.5) 7 (8.4) 0.547
Patient using Mannitol, n(%) 4 (8.3) 0 (0) 0.017
Patient using Aminoglycoside, n(%) 3 (6.3) 2 (2.4) 0.355 Patient using furosemide, n(%) 3 (6.3) 41 (49.3) 0.000 Total dose of used furosemide, mg 3.54±20.36 20.24±30.52 0.000 Mean daily dose of used furosemide, mg/day 1.17±6.77 7.36±10.32 0.000
Disease severity
ISS 31.33±15.08 NA
SAPS3 NA 64.56±11.13
*Overweight = weight-ideal body weight; **Overweight rate = {(weight-ideal body weight)/ideal body weight}*100; ***Ccr, creatinine clearance = [{(140-age)ⅹweight}/(72ⅹCr)], if female ⅹ 0.85; ****[ ], interquartile range
BMI, body mass index; BSA, body surface area; DM, diabetes mellitus; CKD, chronic kidney disease; BUN, blood urea nitrogen; Cr, creatinine; pH, potential of hydrogen; ARB, angiotensin receptor blocker; NSAIDs, nonsteroidal anti-inflammatory drugs; ISS, Injury Severity Score; SAPS3, Simplified Acute Physiology Score 3; NA, not assessed
16
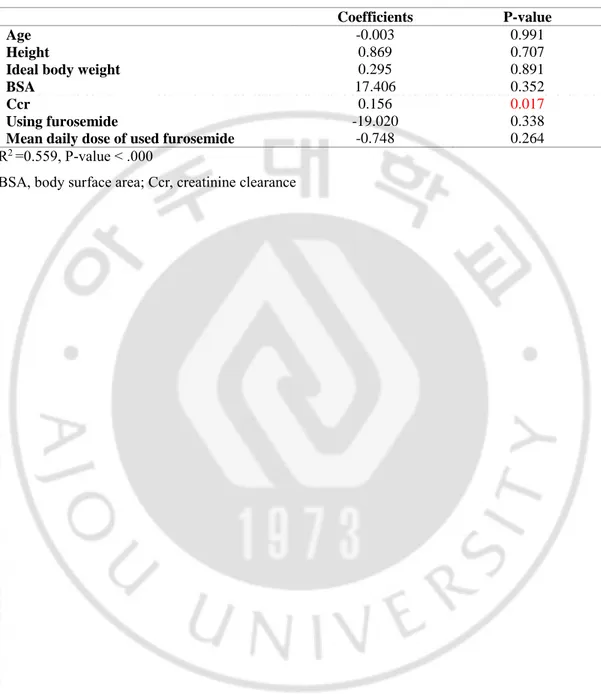
Table 2. Pharmacokinetics of vancomycin in trauma intensive care unit (TICU) and medical intensive care unit (MICU) patients without continuous renal replacement therapy (CRRT) and with CRRT
TICU MICU P-value
Non CRRT (n=126)
Total vancomycin dose, g 5.19±1.81 4.90±1.60 0.347
Daily vancomycin dose, g/day 1.96±0.45 1.91±0.36 0.477
Daily vancomycin dose per weight, mg/kg/day 27.85±5.88 34.71±9.24 0.000
Peak level of vancomycin, μg/ml 25.95±9.95 32.64±10.79 0.001
Trough level of vancomycin, μg/ml 10.42±8.13 14.63±8.47 0.006
Volume of distribution, L 51.12±7.92 45.82±8.70 0.001
Half-life of vancomycin, hr 9.73±6.19 11.63±5.75 0.079
Optiaml vancomycin dose, mg 3251±1647 2094±1101 0.000
Clearance of vancomycin, ml/min 74.25±27.99 53.74±23.91 0.000
CRRT (n=46)
Total vancomycin dose, g 3.42±0.85 3.32±1.94 0.824
Daily vancomycin dose, g/day 1.10±0.25 0.89±0.40 0.046
Daily vancomycin dose per weight, mg/kg/day 13.66±2.65 14.71±8.04 0.652
Peak level of vancomycin, μg/ml 24.98±5.22 28.90±11.73 0.268
Trough level of vancomycin, μg/ml 15.55±4.17 12.38±6.56 0.068
Volume of distribution, L 49.68±7.92 45.24±7.54 0.034
Half-life of vancomycin, hr 19.32±5.13 19.35±3.72 0.986
Recommend vancomycin dose, mg 1088±317 1039±281 0.637
17
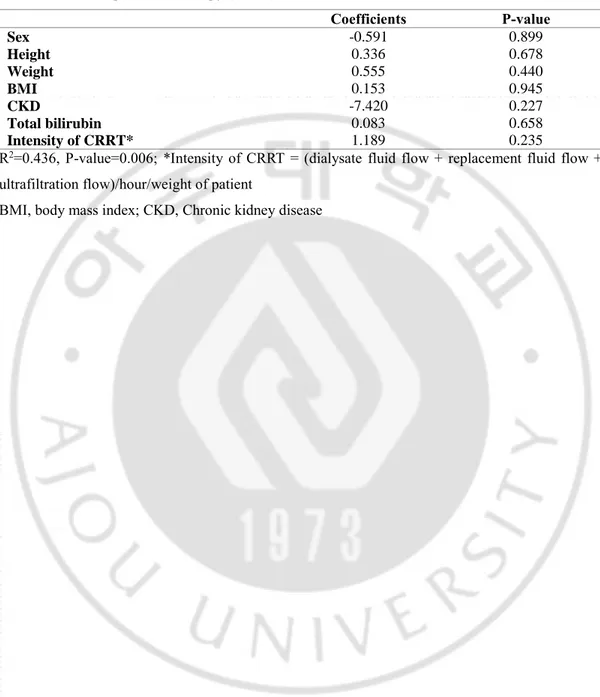
Table 3. Linear regression of factors associated with vancomycin clearance in trauma intensive care unit (TICU) patients without continuous renal replacement therapy (CRRT)
Coefficients P-value
Age -0.003 0.991
Height 0.869 0.707
Ideal body weight 0.295 0.891
BSA 17.406 0.352
Ccr 0.156 0.017
Using furosemide -19.020 0.338
Mean daily dose of used furosemide -0.748 0.264 R2 =0.559, P-value < .000
18
Table 4. Linear regression of factors associated with vancomycin clearance in patients with continuous renal replacement therapy (CRRT)
Coefficients P-value Sex -0.591 0.899 Height 0.336 0.678 Weight 0.555 0.440 BMI 0.153 0.945 CKD -7.420 0.227 Total bilirubin 0.083 0.658 Intensity of CRRT* 1.189 0.235
R2=0.436, P-value=0.006; *Intensity of CRRT = (dialysate fluid flow + replacement fluid flow + ultrafiltration flow)/hour/weight of patient
19
REFERENCES
1. Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care. 2006;12(4):325-32.
2. Sedlar M, Kvasnicka J, Krska Z, Tomankova T, Linhart A. Early and subacute inflammatory response and long-term survival after hip trauma and surgery. Arch Gerontol Geriatr. 2015;60(3):431-6.
3. Bunnell KL, Zullo AR, Collins C, Adams CA, Jr. Methicillin-Resistant Staphylococcus aureus Pneumonia in Critically Ill Trauma and Burn Patients: A Retrospective Cohort Study. Surgical infections. 2017;18(2):196-201.
4. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325-7.
5. McKenzie C. Antibiotic dosing in critical illness. J Antimicrob Chemother. 2011;66 Suppl 2:ii25-31.
6. Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42 Suppl 1:S35-9.
7. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr., Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98.
8. Scaglione F, Paraboni L. Pharmacokinetics/pharmacodynamics of antibacterials in the Intensive Care Unit: setting appropriate dosing regimens. Int J Antimicrob Agents. 2008;32(4):294-301.
9. Pea F, Porreca L, Baraldo M, Furlanut M. High vancomycin dosage regimens required by intensive care unit patients cotreated with drugs to improve haemodynamics following cardiac surgical procedures. J Antimicrob Chemother.
20
2000;45(3):329-35.
10. Baker SP, O'Neill B, Haddon W, Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187-96.
11. Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49(1):1-16.
12. Lin Wu FL, Liu SS, Yang TY, Win MF, Lin SW, Huang CF, et al. A Larger Dose of Vancomycin Is Required in Adult Neurosurgical Intensive Care Unit Patients Due to Augmented Clearance. Ther Drug Monit. 2015;37(5):609-18.
13. Medellin-Garibay SE, Ortiz-Martin B, Rueda-Naharro A, Garcia B, Romano-Moreno S, Barcia E. Pharmacokinetics of vancomycin and dosing recommendations for trauma patients. J Antimicrob Chemother. 2016;71(2):471-9.
14. Choi G, Gomersall CD, Tian Q, Joynt GM, Freebairn R, Lipman J. Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med. 2009;37(7):2268-82.
15. DelDot ME, Lipman J, Tett SE. Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous haemodiafiltration. Br J Clin Pharmacol. 2004;58(3):259-68.
21 국문 요약
외상 집중치료실 환자의 반코마이신 제거율에 영향을 미치는
인자
아주대학교 대학원 의학과 조헌도 (지도교수: 최영화) 연구 배경: 외상으로 집중치료실에 입실한 환자들의 치료에는 반코마이신의 투여할 때 에는 적절한 용량을 결정하는 것이 중요하다. 아주대학교 부속병원에서 환자들의 혈중 반코마이신 최저 농도를 측정하였고, 외상 집중치료실 환자들의 농도가 다른 집중치료 실 환자들의 농도보다 낮음을 확인할 수 있었다. 그리고 이러한 낮은 혈중 반코마이신 농도는 집중치료실 환자들의 치료의 실패를 야기할 수 있다. 우리는 외상 집중치료실 환자들을 내과계 집중치료실 환자들과 비교하여 반코마이신의 약동학에 영향을 미치는 인자들을 확인하고 적절한 반코마이신 투여 용량에 대한 안내를 하고자 한다. 연구 방법: 아주대학교 부속병원의 약제부에서 치료적 약물 농도 감시 자료들에서 적절 한 사례가 후향적으로 수집되었다. 2015년 1월부터 2015년 12월까지 외상 집중치료 실과 내과계 집중치료실에 입실한 환자들이 선택되었다. 모든 환자들은 최소 3회의 반 코마이신이 정맥 투여 되어서 혈중 반코마이신 농도가 항정 상태에 도달하였다고 예상 할 수 있었다. 항정 상태의 환자들에게서 혈중 반코마이신 최저 농도와 치료적 약물 농도 감시가 확인되었다. 19세 미만과 신기능 감소로 인한 간헐적 혈액 투석과 지속적 복막 투석을 하는 경우와 임신한 환자들은 배제되었다. 외상 집중치료실의 56명의 환 자에서 76개의 사례 및 내과계 집중치료실의 89명의 환자에서 96개의 사례가 분석되 었다. 환자들의 과거력 및 혈액 검사, 반코마이신의 사용과 다른 약제의 사용, 임상 소 견과 반코마이신의 약동학이 검토 되었다. 연속 변수는 평균과 표준편차 표현되었으며, 범주형 변수는 횟수와 백분율로 표시되었다. 군간의 비교는 스튜던트 T 검정과 카이22 자승 검정으로 분석되었다. 변수간의 상관성의 평가는 피어슨 검정으로 이루어졌으며, 각 변수간의 개개의 상관성은 다중선형회귀분석으로 분석되었다. 연구 결과: 외상 집중치료실과 내과계 집중치료실 환자들의 많은 특성들이 유의하게 차 이를 보였으며, 특히 체중 (71.93±14.78, 58.36±19.88; kg; P< .0001) 과 이와 관련 된 과체중 과체중 비율, 체질량지수, 체표면적에서 차이가 크며, 크레아티닌 청소율 (143.52±69.41, 97.24±63.69; mg/dl; P< .0001) 에서도 차이가 보였다. 지속적 신 대체요법을 사용하지 않은 군에서는 두 군간의 반코마이신 약동학의 차이가 더욱 컸으 며, 반코마이신 청소율은 외상 집중치료실군에서 더 높았다 (74.25±27.99, 53.74± 23.91; ml/min; P< .0001). 하지만 지속적 신 대체요법을 사용하는 경우에는 반코마이 신 청소율이 두 군간에 유의한 차이를 보이지 않았다 (35.42±11.03, 30.96±9.64; ml/min; P= .215). 반코마이신 청소율에 영향을 미치는 인자는 지속적 신 대체요법을 사용하지 않은 군에서는 크레아티닌 청소율이 있었으며 (coefficients 0.156; P= .017), 지속적 신 대체요법을 사용하는 군에서는 없었다. 연구 결론: 집중치료실에서 치료를 받는 외상 환자들은 집중적인 수액 치료로 인하여 다른 집중치료실 환자들 보다 체중이 많이 나간다. 그렇기 때문에 이러한 환자들에서 환자들의 체중과 크레아티닌 청소율을 고려한 적절한 반코마이신의 용량의 선택과 치 료적 반코마이신 농도의 측정이 필요하다. 지속적 신 대체요법을 사용하는 환자들은 집중치료실 종류나 환자의 특성에 관계 없이 반코마이신을 고정된 용량으로 사용할 수 있겠다. _________________________________________________________________________ 핵심어: 외상 집중 치료실; 반코마이신; 치료적 약물 농도 감시; 크레아티닌 청소율