의
의
의학
학
학 석
석
석사
사
사학
학
학위
위
위 논
논
논문
문
문
F
F
Fa
a
ac
c
ct
t
to
o
or
r
rs
s
sA
Af
A
f
ff
f
fe
e
ec
ct
c
t
ti
i
in
n
ng
g
gN
N
Ne
e
eu
u
ur
r
ro
o
og
g
ge
e
en
n
ne
es
e
s
si
i
is
s
sa
a
af
f
ft
t
te
e
er
r
r
A
A
Ap
p
pp
p
pl
l
li
i
ic
c
ca
at
a
t
ti
i
io
o
on
n
no
o
of
f
fH
H
Hu
u
um
m
ma
a
an
n
nM
M
Me
e
es
s
se
e
en
n
nc
c
ch
h
hy
y
ym
m
ma
a
al
l
l
S
S
St
t
te
e
em
m
mC
C
Ce
e
el
l
ll
l
ls
s
si
i
in
n
nI
I
Is
s
sc
c
ch
h
he
e
em
m
mi
i
ic
c
cR
R
Ra
a
at
t
tM
M
Mo
o
od
d
de
e
el
l
l
아
아
아 주
주
주 대
대
대 학
학
학 교
교
교 대
대
대 학
학
학 원
원
원
의
의
의 학
학
학 과
과
과
이
이
이 문
문
문 옥
옥
옥
F
F
Fa
a
ac
c
ct
t
to
o
or
r
rs
s
sA
Af
A
f
ff
f
fe
e
ec
ct
c
t
ti
i
in
n
ng
g
gN
N
Ne
e
eu
u
ur
r
ro
o
og
g
ge
e
en
n
ne
es
e
s
si
i
is
s
sa
af
a
f
ft
t
te
e
er
r
r
A
A
Ap
p
pp
p
pl
l
li
i
ic
c
ca
a
at
t
ti
i
io
o
on
n
n o
o
of
f
fH
H
Hu
u
um
m
ma
an
a
n
n M
M
Me
e
es
s
se
e
en
n
nc
c
ch
hy
h
y
ym
m
ma
a
al
lS
l
S
St
t
te
e
em
m
m
C
C
Ce
e
el
l
ll
l
ls
s
si
i
in
n
nI
I
Is
s
sc
c
ch
h
he
e
em
m
mi
i
ic
c
cR
R
Ra
a
at
t
tM
M
Mo
o
od
d
de
e
el
l
l
by
by
by
by
Wen
Wen
Wen
Wen Yu
Yu
Yu
Yu Li
Li
Li
Li
A A AA Dissertation Dissertation Submitted Dissertation Dissertation Submitted Submitted Submitted to to to to The The The Graduate The Graduate School Graduate Graduate School School of School of of of Ajou Ajou Ajou UniversityAjou UniversityUniversityUniversity in
in in
in Partial Partial Partial Partial Fulfillment Fulfillment Fulfillment Fulfillment of of of of the the the Requirements the Requirements for Requirements Requirements for for for the the the Degree the Degree Degree ofDegree ofofof
MASTER MASTER MASTER MASTER OF OF OF MEDICAL OF MEDICAL MEDICAL MEDICAL SCIENCESSCIENCESSCIENCESSCIENCES Supervised Supervised Supervised Supervised bybybyby Kyoon Kyoon Kyoon Kyoon Huh, Huh, Huh, M.D. Huh, M.D. M.D. M.D.
Department Department Department
Department of of of Medical of Medical Medical Medical SciencesSciencesSciencesSciences The
The The
The Graduate Graduate Graduate Graduate School, School, School, School, Ajou Ajou Ajou Ajou UniversityUniversityUniversityUniversity February, February, February, 2006 February, 2006 2006 2006
이
이
이문
문
문옥
옥
옥의
의 의
의
의
의학
학
학 석
석
석사
사
사학
학
학위
위
위 논
논
논문
문
문을
을 인
을
인
인준
준
준함
함
함.
.
.
심
심
심사
사
사위
위
위원
원
원장
장
장
허
허
허
균
균
균
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
주
주
주 인
인
인 수
수
수
인
인
인
심
심
심 사
사
사 위
위
위 원
원
원
방 오
방
방
오
오 영
영
영
인
인
인
아
아
아 주
주
주 대
대
대 학
학
학 교
교
교 대
대
대 학
학
학 원
원
원
2
2
20
0
00
0
05
5
5년
년
년 1
1
12
2
2월
월
월 2
2
22
2
2일
일
일
i
- ABSTRACT -
Factors Affecting Neurogenesis after Application of Human
Mesenchymal Stem Cells in Ischemic Rat Model
Background: We recently reported the results of clinical trial of ex vivo culture
expanded autologous mesenchymal stem cells (MSCs) in patients with severe ischemic stroke. We proposed that an optimal condition, for either donor or recipient, may exist and enhance the effects of the mesenchymal stem cells in those patients. Thus we evaluated the effect of the MSCs in impacts on the neurogenesis depending on the passage of MSCs.
Methods and Results: We performed immunohistochemical studies and
behavioral tests in transient middle cerebral occlusion rat model. Intravenous application of ex vivo cultured human mesenchymal cells, earlier (passage 2) or later (passage 6), was performed in stroke rat model. Compared to rat received later passage MSCs, neurogenesis revealed by bromodeoxyuridine staining was more pronounced in rat received early passage MSCs (P<0.05). Double staining showed that neuroblast (bromodeoxyuridine positive cells), but not MSCs (human nuclei matrix antigen positive cells), expressed neuronal and glial phenotypes.
Conclusions: Our results indicated that ischemia-induced neurogenesis was
enhanced by the application of mesenchymal stem cells, this process was related to the passage of ex vivo stem cell culture.
ii
____________________________________
Key words: human mesenchymal stem cell; stroke; neurogenesis; passage
iii TABLE OF CONTENTS TABLE OF CONTENTS TABLE OF CONTENTS TABLE OF CONTENTS ABSTRACT --- i
TABLE OF CONTENTS --- iii
LIST OF FIGURES --- --- iv
LIST OF TABLES --- v
I. INTRODUCTION --- 1
II. MATERIALS AND METHODS --- 3
A. Animal MCAo model --- 3
B. Experimental Groups --- 3
C. hMSC culture --- 4
D. Cell baleling --- 4
E. Functinal tests --- 5
F. Histological and immunohistochemical assessment --- 6
1. 2,3,5-triphenyltetrazolium chloride(TTC) staining --- 6
2. Immunohistochemistry --- 7
G. BrdU-labeled cell count in SVZ and ischemic border zone --- 9
III. RESULTS --- 10 IV. DISCUSSION --- 12 REFERENCES --- 22 국문요약 국문요약 국문요약 국문요약 --- 27
iv
LIST OF FIGURES
Fig. 1. TTC stained coronal brain sections --- 17 Fig.2. hMSC labeled in 14d after ischemia rat --- 17
Fig.3. 14d after post-MCAo brain section labled for
SDF-1 in the border zone --- 18
Fig.4. Results of behave functional tests --- 18
Fig.5. Neurogenesis.BrdU immunostaining in the SVZ of the ipsilateral
hemisphere at the 14d after each group --- 19
Fig.6. Phenotype of BrdU-labeled cells in border
zone at 14d after MCAO --- 20
v
LIST OF TABLES
Table1. Modified neurologic severity score test --- 16
1
I. INTRODUCTION
The use of mesenchymal stem cells (MSCs) as therapy for stroke is attractive. MSC therapy has already been used to treat patients with cancer. Moreover, it is conceivable that autologous MSCs could be used, which would allow immune reactions to be avoided. Recently, the transplantation of bone marrow mononuclear cells (mainly hematopoietic stem cells) achieved clinical efficacy by inducing angiogenesis in patients with myocardial infarction (Strauer et al., 2002; Tse et al., 2003) or limb ischemia (Tateishi-Yuyama, et al., 2002).
MSCs have recently been investigated for their efficacy as a clinical therapeutic tool. The multi-lineage potential of MSCs has been under intense scrutiny in recent years due to documented transplantation success in treating animal disorders (Young et al., 1998; Jin et al., 2002; Shake et al., 2002; Vacanti et al., 2005). Transplantation of bone marrow cells into cerebral ischemia models (Chen et al., 2001; Chen et al., 2001; Kurozumi et al., 2004; Li et al., 2002) has demonstrated reduced lesion size and improved functional outcome. However, mechanisms underlying the beneficial effects of these therapies have not been fully investigated. MSCs promote endogenous plasticity, angiogenesis and neurogenesis (Chen et al., 2003; Zhang et al., 2001; Zhang et al., 2003). Functional benefit derived from these cells, derives either from their ability to differentiate or integrate into cerebral tissue and act as stem or progenitor cells (Chen et al., 2001; Deng et al., 2005), from their ability to induce endogenous restorative activity, by evoking the production of neurotrophic factors,
2
such as BDNF, and bFGF (Chen et al., 2000; Wada et al., 2003), or from both mechanisms. On the cell therapeutic forefront, the capacity for large scale expansion of MSCs has facilitated the development of clinical trials aimed at assessing the safety and efficacy of MSC transplantation for a variety of pathological conditions.
We recently reported the results of clinical trial of autologous mesenchymal stem cells (MSCs) in patients with severe ischemic stroke (Bang et al., 2005). Transplantation after ex vivo culture expansion of MSCs is mandatory to meet the dose requirements that have been effective in animal models, because few MSCs can be obtained by bone marrow aspiration. Our preliminary results have shown that it is feasible and perhaps safe to administer ex vivo–cultured autologous MSCs intravenously in patients with ischemic stroke. One of the key questions to be solved with the intravenous administration of MSCs is what would be the best cell dose and best timing to perform the intervention (Savitz et al., 2002; Bang, 2005). We proposed that an optimal condition, for either donor or recipient, may exist and enhance the effects of MSCs in those patients.
The aim of this study was to determine whether enhance of hMSC treatment on ischemia brain is related to the characteristics of MSCs (their passage-related proliferation ability) or the condition of recipient brain (the expression of chemokines). Thus we analyzed the differential effects of hMSCs in impacts on the neurogenesis depending on their passage and chemokine expression. In addition, we performed immunohistochemical study to evaluate the possible mechanisms of effects after the application of ex vivo cultured MSCs.
3
MATERIALS AND METHODS
A. Animal model
Anesthesia was induced in male Sprague–Dawley rats (250–300 g) with 4% isoflurane and maintained with 1.5% isoflurane in 70% N2O and 30% O2 using a face
mask. Rectal temperature was maintained at 37.0–37.5°C with heating pads. We induced transient middle cerebral artery occlusion (MCAo) using a method of intraluminal vascular occlusion modified in our laboratory (Chen et al., 1992; Chen et al., 2000). A 4–0 surgical monofilament nylon suture with rounded tip from the left common carotid artery into the lumen of the internal carotid artery to block the origin of the MCA, 2 h after MCAo, animals were performed by withdrawal of the suture until the tip cleared the lumen of the CCA.
B. Experimental Groups
One day after MCAo animal were randomly divided into four groups: (a) sham ischemia + phosphate buffered saline (PBS) injection (n=6), (b) ischemia + PBS injection (n=6), (c) ischemia + 2nd passages (P2) human MSC (hMSC) (1x106, n=6) injection, and (d) ischemia + 6th passages (P6) hMSC (1x106, n=6) injection. All animals received daily intraperitoneal (i.p.) injections of bromodeoxyuridine (BrdU, a thymidine analog, which labels newly synthesized DNA (Miller and Nowakowski, 1998, 50 mg/kg, Roche) starting at 24 h after MCAo and subsequently for 13 days consecutive days for identification of endothelial cell proliferation. All animals were sacrificed at 14 days after MCAo.
4
C. hMSC culture
hMSCs were obtained from 20 mL aspirates from the iliac crest of normal human donors (Bang et al., 2005; Chen et al., 2003; Li et al., 2002). Each 20 mL of aspirate was diluted 1:1 with phosphate buffered saline (PBS) and layered over 10 mL of Ficoll (Ficoll-Paque; Amersham Biosciences). After centrifugation at 2000 rpm for 20 minutes, the mononuclear cell layer was removed from the interface and suspended in PBS. Cells were centrifuged at 1200 rpm for 5minutes and resuspended in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS. The cells were incubated at 37°C in 5% CO2 in flasks for 1 day and non-adherent cells
were removed by replacing the medium. After the cultures reached confluence, usually 1 to 2 weeks, the cells were harvested with 0.05% trypsin and 0.53 mmol/L EDTA (GIBCO) for 5 minutes at 37°C, replated in a flask, again cultured for about 2 weeks, and harvested. They were then frozen for later use. Cells used in these experiments were harvested at 2 and 6 passages. The protocol was approved by the Institutional Review Board of Ajou University hospital.
D. Cell labeling
At the end of the expansion period, cells were labeled with the fluorescent dye PKH26 (Sigma) (Chen et al., 2005; Krause et al., 2001). The labeling procedure was performed according to the manufacturer’s protocol with some modifications to optimize the procedure for MSCs. Different concentrations from 2×10−5 M to 2×10−6 M (suggested by the manufacturer) were used. Briefly, after trypsinization, 2×107
5
MSCs were washed twice in calcium-free and magnesium-free PBS and resuspended into 1 ml of the dilution buffer provided by the manufacturer in the labeling kit. This cell suspension was mixed with an equal volume of the labeling solution containing PKH26 in the dilution buffer to give the desired final concentration. Labeling was allowed for 4 min at room temperature with periodical tapping of the tube. The reaction was terminated by adding 2 ml FBS. Cells were washed twice in 4 ml DMEM, and the labeling ratio was counted under a fluorescent microscope.
E. Functional tests
In all animals, a battery of behavioral tests was performed before MCAo, and at 1, 7 and 14 days after MCAo, by an investigator who was blinded to the experimental groups. For adhesive-removal somatosensory test (Chen et al., 2001; Chen et al., 2004; Li et al., 2002), two small pieces of adhesive-backed paper dots (of equal size, 113.1 mm2) are used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The time to remove each stimulus from forelimbs is recorded on five trials per day. Before surgery, the animals are trained for 3 days. If the rats are able to remove the dots within 10 s, they were subjected to MCAo. The time to remove the dot is recorded. Using modified Neurological Severity Score (mNSS), neurological function was graded on a scale of 0-18 (normal score, 0; maximal deficit score, 18). The mNSS is a composite of the motor (muscle status and abnormal movement) (Borlongan et al., 1995; Schallert et al., 1997), sensory (visual, tactile, and proprioceptive) (Markgraf et al.,1992), reflex (Germano et al.,1994;
6
Schallert et al., 1997), and balance tests (Li et al., 2002) (Table 1).
An accelerating rotarod was used to measure motor function of rats. Rats were placed on rungs of the accelerating rotarod and the time the animals remained on the rotarod was measured. The speed was increased slowly from 4 rev/min to 40 rev/min. A trial ended if the animal fell off the rungs or gripped the device and spun around for two consecutive revolutions without attempting to walk on the rungs. The animals were trained by five trials per day for 3 days before MCAo to obtain stable baselines. For each rat, the control preischemia data — i.e., mean duration (in s) on the device — was recorded from three test trials performed immediately prior to MCAo. The motor test data are presented as percentage of mean duration (three trials before sacrifice) compared with the internal control.
F. Histological and immunohistochemical assessment
1. 2, 3, 5-triphenyltetrazolium chloride (TTC) staining
TTC stain provides a faster and simpler approach for differentiation of ischemic damage area from healthy region. TTC reacts with intact mitochondrial oxidative enzyme systems and when reduced becomes a deep red colored formazan. In ischemic tissue damaged mitochondria with disturbed oxidative systems (Callaway et al., 2000). Infarct brain tissue usually demonstrates a white or pink color embedded in the healthy brain tissue of deep red color with TTC stain.
In brief, 24 h after MCAo, rats were anesthetized with chloral hydrate. The brains were removed immediately sectioned coronally into six slices (2-mm thick) in a
7
rodent brain matrix (Harvard Instrument Inc., South Natick, MA). Brain slices were placed in 2% TTC (T-8887, Sigma chemical, Steinheim, Germany) under dark conditions and incubated 37℃ for 40min. The brain slices were then removed from the incubator. TTC stained out and replaced with 10% formalin. The stained sections were photographed and scanned with a flatbed color saner (Khan et al., 2000; Wang et al., 2001).
The infarct was mostly located with the ipsilateral cortex and striatum (Fig. 1)
2. Immunohistochemistry.
Animals were sacrificed at 14 days after MCAo. Rat brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde solution. The brains were quickly removed and kept in 4% paraformaldehyde solution overnight at 4℃, and then embedded in 30% sucrose solution until they sunk. Coronal section (30㎛ thick) were cut using a cryostat (Leica, CM1800, Germany) for immunohistochemical staining.
For immunohistochemisty the section were washed three times with PBS and nonspecific binding was blocked with 10% normal horse serum; cell derived from hMSC were identified using morphologic criteria and immunohistochemical staining with NuMA (human nuclei matrix antigen; Oncogen) present in the donor cells but not present in the parenchymal cells. To confirm whether the chemokine SDF-1 was localized within specific cells in the ischemic brain regions, another sections were labeled with antibodies to SDF-1 (goat, 1:100, Santa Cruz). SDF-1 signal was
8
detected FITC conjugated antibody.
In order to make it clear whether BrdU-labeled cells differentiate into neuronal or astroglial cells, we performed immunohistochemistry with the use of antibodies for BrdU, glial fibrillary acidic protein (GFAP for astrocytes; Sigma), neuronal nuclear protein (NeuN for neuron) or neurofilament subunit (NF-H for neuron), and doublecortin (DCX C-18 for migrating neurons). Sections were treated with 2N HCl and rinsed as above, blocked with 10% normal horse or Goat serum and 0.1% Triton X-100 in PBS for 30 mins at room temperature, and incubated with rat monoclonal anti-BrdU overnight at 4℃. After being rinsed with primary antibodies, sections were incubated for 1 h with cy3-conjugated goat anti-rat IgG (1:400, abcom). Section were then incubated with anti-NF-H, anti-NeuN,anti-DCX and anti-GFAP overnight at 4℃; incubated with anti-rabbit Alxa 488, or anti-mouse Alxa 488 antibody for 1 h at room temperature. Section were washed in PBS, rinsed with double-distilled water and mounted onto slides using an anti-fade mounting medium (Permafluor, Molec, Probes). Specimens were examined on a zeiss LSM510 confocal imaging system.
To evaluate the degree of neurogenesis, BrdU positive cells were counted. To do this, DNA was first denatured by incubating brain sections (30㎛) in 2N HCl at 37°C for 1 h. Sections were then rinsed with Tris buffer and treated with 0.3% of H2O2 to
block endogenous peroxidase. Section were blocked with 10% normal horse serum and 0.1% Triton X-100 in PBS for 30 min at room temperature and incubated with a mouse monoclonal antibody (mAb) against BrdU (1:200, Roche) overnight at 4℃. Section were then incubated with horse anti-mouse IgG for 1 h at room temperature
9
and with avidin-biotin- peroxidase complex (vector laboratory) for 1h at room temperature and developed in diaminobenzidine-H2O2. Section were mounted on
glass slides and examined with on Olympus BH-2 microscope.
G. BrdU-Labeled cell count in SVZ and ischemic border zone
To determine the number of BrdU-labeled cells in the SVZ of the ipsilateral hemisphere, every 12th section (each 30㎛) between bregma levels +1.0 and -1.0 mm were selected (total 6 section per brain). For quantitatively of BrdU-labeled cells (Hayashi et al., 2005; Wada et al., 2003), we counted the positive stained cells in SVZ and ischemic border zone (Fig. 5A). Result were expressed as mean ± SD, and compared between the sham-control brains and MCAo only groups.
10
III. RESULTS
A. Cell labeling
For in vivo cell tracking of hMSCs, we selected PKH26 as the labeling system. The recommended concentration for the fluorescence label PKH26 proved to be effective. Satisfactory “long-term” cell labeling was achieved by optimizing the dye concentration to 2×10−6 M in vivo conditions. Over 95% of MSCs were evenly labeled by PKH26 (Fig. 2C).Cells derived from hMSC were identified by the human specific antibody NuMA. hMSC survived and were distributed throughout the ischemic damaged brain of recipient rats. Although NuMA-reactive cells were observed in multiple areas of the ipsilateral hemisphere, including the cortex and striatum, most NuMA-labeled hMSC were located in the ischemic boundary zone (Fig. 2B).
B. Neurologic functional testing
At 14 day after MCAo, functional recovery shown by the adhesive-removal test, mNSS test and rotarod test was found in rats injected with P2 and P6 hMSCs compared with control MCAo rats. Although there was trend that rat received P2 showed better improvement than rats received P6 MSCs, no significant difference was noted in the behavioral recovery of animals that P2 or P6 hMSC injected MCAo rats (Fig. 4).
11
C. Cell proliferation of SVZ in the ischemia brain
The data showed that, in the ipsilateral SVZ, BrdU positive cells were significantly increased in the MCAo + P2 hMSCs (Fig. 5. left C) and MCAo + P6 hMSCs (Fig. 5. left D) compared to the MCAo + PBS treatment group (Fig. 5. left B) and sham group (Fig. 5. left A) (P<0.05). Significantly more BrdU immunoreactive cells were found in the P2 hMSC-treated group compared to other groups. The BrdU positive cells were marginally increased in the P6 hMSC-treated group compared to MCAo + PBS-treated group (Fig. 5. Right C and D). These data demonstrate that P2 hMSC treatment promotes complementary effect on neurogenesis.
To investigate whether such differences in neurogenesis after application of hMSCs were caused by the donor brain conditions, such as chemokine expression, SDF-1 immunostaining was performed (Fig. 3. A and B). SDF-1 positive cells increased ischemic border zone. No significant difference was found between MCAo only group and hMSC-injected groups (P2 and P6).
D. The origin of central nervous system type cells
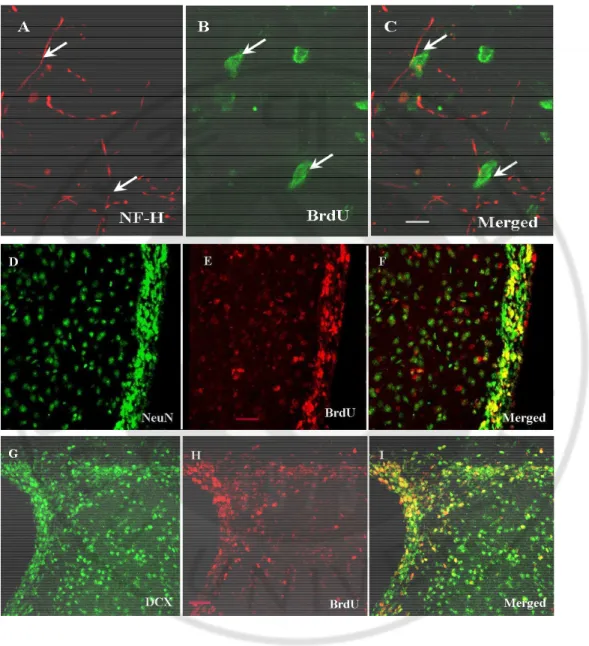
Double staining indicated that some BrdU positive cells colocalized with NF-H, NeuN, DCX and GFAP (Fig. 6. C, G, J and M). However, no NuMA positive cells expressed either neuronal or glial phenotype (Fig 6. L). In addition, BrdU positive cells, but not NuMA positive cells, expressed doublecortin, a marker of migrating neuroblast.
12
IV. DISCUSSION
A. Mode of actions of hMSCs
Compared with rats subjected to MCAO alone, IV injection of hMSCs 1 day after stroke significantly improved functional outcome according to the adhesive-remove test, mNSS, and rotarod test. Controversy surrounds the question of whether true transdifferentation (Cogle et al., 2004; Tondreau et al., 2004), spontaneous cell fusion (Terada et al., 2002; Ying et al., 2002), or trophic supports (Chen et al. (a), 2001) are the primary cause for the improvement in the functional outcome after application of MSCs in animal model for ischemic stroke. Our immunohistochemisty results indicate that endogeneous neuronal progenitor cells (BrdU positive), but not hMSCs (NuMA positive), expressed neuronal or glial phenotype. Our data suggest that, rather than transdifferentiation, upregulation of endogenous recovery mechanism either at the peri-infarct area (neurogenesis) or at area that are remote from the infarct (neuronal plasticity) were important role of hMSCs in the functional recovery after ischemic stroke. This benefit may reflect production of growth factors, including neurotrophins that may promote repair of damaged parenchymal cells, reduce apoptosis in the ischemic boundary zone, and enhance proliferation and differentiation of endogenous neural stem and progenitor cells in the SVZ after stroke in rats (Li et al., 2002). Supporting this hypothesis, a recently study reported that hMSCs have the capacity to secrete BDNF, NGF, VEGF and HGF in culture and that hMSCs are sensitive to the normal and ischemic injured brain environment and
13
respond through an increased production of these neurotrophins and angiogenic factors (Chen et al., 2002). The ability of MSCs to secrete multiple growth factors and cytokines is suggestive of their critical trophic roles in a plethora of cellular and physiological functions (Majumdar et al., 1998 and 2000; Wieczorek et al., 2003).
B. Passage of hMSCs as a Factor Affecting the Efficacy of Stem Cell Therapy
1. Passage of hMSCs and neurogenesis
Our data demonstrate that hMSC + P2 hMSC treatment promotes neurogenesis in the SVZ and IBZ and that such effect was more pronounced in the earlier passage MSCs (P2) than in the later passage (P6). Although there have been several studies concerning the growth pattern of MSCs (Zhang et al., 2005; Liu et al., 2003), Zhang et al. recently reported that the population doubling time of P4 rat MSCs was prolonged to nearly 3-fold compared to that on P1 and that, in contrast to the rapid proliferation phase in P1 rat MSCs, P4 rat MSCs entered a growth arrest phase
(Zhang et al., 2005). The similar growth pattern of rat MSC, such as rapid
proliferation at P1, slow growth at P3, and growth arrest at P4, were observed by Liu et al (Liu et al., 2003). These results suggest that the growth properties of different passage hMSCs might be one of the factors determining the neurogenesis in the ischemic rat brain, But there was no report comparing the effects of MSCs on neurogenesis after stroke depending on their passage.
14
Our hypothesis was that the characteristics of hMSCs (such as passage numbers) may be important in consideration of hMSCs for enhancement of the ischemia-induced neurogenesis. Our data showed that neurogenesis was enhanced by the application of hMSCs and that such effects were more prominent in earlier passage of hMSCs than in the later passage. Thus it is conceivable that in the clinical application of hMSCs it might be better to apply a smaller dose of early passage of hMSCs rather than delay to achieve more numbers of hMSCs of late passage with further ex vivo cultivation.
However, further studies comparing smaller dose of early passages and the larger dose of late passages because it was reported that animals with ischemia-induced brain damage infused with a high dose of MSCs (3×106) recovered better than did control animals infused with a low dose of MSCs (1×106) (Chen et al.(a), 2001). In this present study, no significant difference was noted in the behavioral recovery of animals that P2 and P6 hMSC injected MCAo rats. Further studies are needed to decide the ideal passage and numbers of hMSCs.
2. Passage of hMSCs and chemokine expression
The chemokine SDF-1 (also known as CXCL12) and its receptor CXCR4 have been implicated in homing of stem cells to the bone marrow and the homing of bone marrow-derived cells to sites of injury. It was reported that SDF-1 protein expression was as detected by anti-SDF-1 is clearly upregulated in the infarcted hemisphere within 24 hours and it is maintained through at least 30 days post-occlusion (Hill et
15
al., 2004). Thus, with respect to the recipient brain condition, earlier application of hMSCs may be important because there exist the possibility that the homing effects of SDF-1 to injured area diminish with time (Abbott et al., 2004).
Our results of the prominent neurogenetic potentials of earlier passage of MSCs compared to that of the later passage MSCs are unlikely caused by the difference in the chemokine levels; our immunohistochemical study of SDF-1 showed that the chemokine levels of the rat were not different between the P2- and P6 MSCs received rat.
C. Conclusions
In conclusion, we demonstrate that MSCs derived from adult bone marrow can enhance neurogenesis in the ischemia rat brain and this potential is restricted by proliferation ability and cell passages.
16
Table 1. Modified neurologic severity score test.
Motor tests Points
• Raising the rat by the tail 1=Flexion of forelimb
1=Flexion of hindlimb
1=Head moved >10 degree vertical axis within 30s
3
• Walking on the floor (normal=0, maximum=3) 0=Normal walk
1=Inablility to walk straigh 2=Circling toward the paretic side 3=Fall down to the paretic side
3
• Sensory tests 1=placing test (visual & tactile test)
1=proprioceptive(deep sensation, pushing paw against the table edge to stimulate limb muscles)
2
• Beam balance test (normal=1;maximum=6) 0= balance with steady posture
1= balance with steady posture grasps side of beam 2= hugs the beam & 1 limb falls down from the beam 3= 2 limbs fall down from the beam or spins on the beam 4= attempts to balance on the beam but falls off>40s 5= attempts to balance on the beam but falls off>20s 6=falls off: no attempt to balance or hang on the beam<20
6
• Reflexes and abnormal movements 1=pinna reflex(a head shake when touching the auditory meatus)
1=corneal reflex(an eye blink when lightly ouching the cornea with cotton) 1=startle reflex(a motor response to a brief noise from snapping a clipboard paper)
1=seizures, myoclonus, myodystrophy
4
Maximum points 18 One point is awarded for the inability to perform the tasks or for the lack of a tested reflex.13-18 =sever injury; 7-12 = moderate injury; 1-6 = mild injury.
17
Figure 1. TTC stained coronal brain sections. The images of brain section were
captured by scanner. Brain slides (2mm Thick) stained by the TTC solution 1 day
after MCAo in adult rat. The lesion of cerebral infarct was visualized as the white arer in contrast to the normal red-stained area. The development of the MCAo was
limited in the MCA territory.
Figure 2. hMSC labeled in 14d after ischemia rat. Marked increase in
NuMA-positive MSCs (B) and PKH2-labeled hMSC (C) in the ischemic border zone. Scar bar 20 ㎛ in B and 100 ㎛
18
Figure 3. Fifteen days after post-MCAo brain section labeled for SDF-1 in the
border zone. SDF-1 labeled in the ischemia border zone (A and C) than in the
contralateral zone (B). Scar bar 100㎛. B. scar bar 20㎛
Figure 4. Results of behave functional tests.
(A) Adhesive-remove test ; (B) Modified neurologic severity score [mNSS] test; and (C) Rotarod test before and after middle cerebral artery occlusion (MCAo).
Group 1: MCAo alone(n=4); group 2: intravenous infusion P2 hMSC; or group 3: intravenous infusion P6 hMSC.
19
Figure 5. Neurogenesis.
BrdU immunostaining in the SVZ of the ipsilateral hemisphere at the 14d after each group (Left); (A) Sham operation, (B) tMCAO + PBS, (C) tMCAO + MSC P2, and (D) tMCAO + MSC P6.
BrdU-labeled cells (Right); In the SVZ and penumbra zone, the numbers of labeled cells were largest in P2 hMSC.
20
Figure 6. Phenotype of BrdU-labeled cells in border zone at 14d after MCAO.
(Upper lane) BrdU labeled cells were merged with NF-H( C ), NeuN (F),DCX (I) and GFAP (L). (Lower lane) No NuMA positive cells expressed GFAP (O).
Section were stained for NF-H, NeuN, DCX and GFAP(A,D,G,J) and BrdU (B,E,H,K); NuMA( N) and GFAP(M) were examined by confocal microscopy. A-O Scale bar 20㎛.
22
REFERENCES
Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004 Nov 23;110(21):3300-5.
Bang O. Y., Lee, J. S., Lee, P. H., and Lee, G.. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57: 874-882. 2005.
Bang O.Y. Reply. Ann Neurol 58: 654-655. 2005.
Borlongan, C. V., Randall, T. S., Cahill, D. W., and Sanberg, P. R. Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res 676: 231-234. 1995.
Callaway, J. K., Knight, M. J., Watkins, D. J., Beart, P. M., Jarrott, B., and Delaney, P. M. A novel, rapid, computerized method for quantitation of neuronal damage in a rat model of stroke. J Neurosci Methods 102: 53-60. 2000.
Chen, H., Chopp, M., Zhang, Z. G., and Garcia, J. H. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 12: 621-628. 1992.
Chen, J., Li, Y., and Chopp, M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology 39: 711-716. 2000.
Chen, J., Li, Y., Wang, L., Lu, M., Zhang, X., and Chopp, M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci 189: 49-57. 2001. (b)
Chen, J., Li, Y., Wang, L., Zhang, Z., Lu, D., Lu, M., and Chopp, M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32: 1005-1011. 2001. (a)
Chen, J., Li, Y., Zhang, R., Katakowski, M., Gautam, S. C., Xu, Y., Lu, M., Zhang, Z., and Chopp, M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis.
Brain Res 1005: 21-28. 2004.
Chen, J., Wang, C., Lu, S., Wu, J., Guo, X., Duan, C., Dong, L., Song, Y., Zhang, J., Jing, D., Wu, L., Ding, J., and Li, D. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res 319:
429-23
438. 2005.
Chen, J., Zhang, Z. G., Li, Y., Wang, L., Xu, Y. X., Gautam, S. C., Lu, M., Zhu, Z., and Chopp, M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 92: 692-699. 2003.
Chen, X., Li, Y., Wang, L., Katakowski, M., Zhang, L., Chen, J., Xu, Y., Gautam, S. C., and Chopp, M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 22: 275-279. 2002.
Cogle CR, Yachnis AT, Laywell ED, et al. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet;363:1432–1437. 2004.
Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal Stem Cells Spontaneously Express Neural Proteins in Culture, and Are Neurogenic After Transplantation. Stem Cells.; [Epub ahead of print] 2005.
Germano, A. F., Dixon, C. E., d'Avella, D., Hayes, R. L., and Tomasello, F. Behavioral deficits following experimental subarachnoid hemorrhage in the rat. J
Neurotrauma 11: 345-353. 1994.
Hayashi, T., Iwai, M., Ikeda, T., Jin, G., Deguchi, K., Nagotani, S., Zhang, H., Sehara, Y., Nagano, I., Shoji, M., Ikenoue, T., and Abe, K.. Neural precursor cells division and migration in neonatal rat brain after ischemic/hypoxic injury. Brain
Res 1038: 41-49. 2005.
Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 63(1):84-96. 2004.
Jin, H. K., Carter, J. E., Huntley, G. W., and Schuchman, E. H.. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. J
Clin Invest 109: 1183-1191. 2002.
Khan, S. H., Baziany, A., Banigesh, A., Hemmings, S. J., and Shuaib, A. Evaluation of an optimal temperature for brain storage in delayed 2, 3,5-triphenyltetrazolium chloride staining. J Neurosci Methods 98: 43-47. 2000.
Krause, D. S., Theise, N. D., Collector, M. I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S., and Sharkis, S. J. Multi-organ, multi-lineage engraftment by a single
24
bone marrow-derived stem cell. Cell 105: 369-377. 2001.
Kurozumi, K., Nakamura, K., Tamiya, T., Kawano, Y., Kobune, M., Hirai, S., Uchida, H., Sasaki, K., Ito, Y., Kato, K., Honmou, O., Houkin, K., Date, I., and Hamada, H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol
Ther 9: 189-197. 2004.
Li, Y., Chen, J., Chen, X. G., Wang, L., Gautam, S. C., Xu, Y. X., Katakowski, M., Zhang, L. J., Lu, M., Janakiraman, N., and Chopp, M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59: 514-523. 2002.
Liu, Y., Song, J., Liu, W., Wan, Y., Chen, X., and Hu, C. Growth and differentiation of rat bone marrow stromal cells: does 5-azacytidine trigger their cardiomyogenic differentiation? Cardiovasc Res 58: 460-468. 2003.
Majumdar, M. K., Thiede, M. A., Haynesworth, S. E., Bruder, S. P., and Gerson, S. L. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 9: 841-848. 2000. Majumdar, M. K., Thiede, M. A., Mosca, J. D., Moorman, M., and Gerson, S. L.
Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 176: 57-66. 1998.
Markgraf, C. G., Green, E. J., Hurwitz, B. E., Morikawa, E., Dietrich, W. D., McCabe, P. M., Ginsberg, M. D., and Schneiderman, N. Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res 575: 238-246. 1992.
Miller, M. W., and Nowakowski, R. S. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res 457: 44-52. 1988.
Savitz SI, Rosenbaum DM, Dinsmore JH, et al. Cell transplantation for stroke. Ann Neurol;52:266 –275. 2002
Schallert, T., Kozlowski, D. A., Humm, J. L., and Cocke, R. R. Use-dependent structural events in recovery of function. Adv Neurol 73: 229-238. 1997.
25
M., Pittenger, M. F., and Martin, B. J. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac
Surg 73: 1919-1925; discussion 1926. 2002.
Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106: 1913–1918. 2002
Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 360: 427–435. 2002
Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416:542–545. 2002.
Tondreau T, Lagneaux L, Dejeneffe M, et al. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation 72:319–326. 2004.
Tse HF, Kwong YL, Chan JK, et al. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 361:47– 49. 2003.
Vacanti, V., Kong, E., Suzuki, G., Sato, K., Canty, J. M., and Lee, T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol 205: 194-201. 2005.
Wada, K., Sugimori, H., Bhide, P. G., Moskowitz, M. A., and Finklestein, S. P. Effect of basic fibroblast growth factor treatment on brain progenitor cells after permanent focal ischemia in rats. Stroke 34: 2722-2728. 2003.
Wang, C. X., Yang, Y., Yang, T., and Shuaib, A. A focal embolic model of cerebral ischemia in rats: introduction and evaluation. Brain Res Brain Res Protoc 7: 115-120. 2001.
Wieczorek, G., Steinhoff, C., Schulz, R., Scheller, M., Vingron, M., Ropers, H. H., and Nuber, U. A. Gene expression profile of mouse bone marrow stromal cells determined by cDNA microarray analysis. Cell Tissue Res 311: 227-237. 2003.
Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature 416:545–548. 2002
26
Young, R. G., Butler, D. L., Weber, W., Caplan, A. I., Gordon, S. L., and Fink, D. J. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J
Orthop Res 16: 406-413. 1998.
Zhang, F. B., Li, L., Fang, B., Zhu, D. L., Yang, H. T., and Gao, P. J. Passage-restricted differentiation potential of mesenchymal stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun 336: 784-792. 2005. Zhang, R., Wang, L., Zhang, L., Chen, J., Zhu, Z., Zhang, Z., and Chopp, M. Nitric
oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res 92: 308-313. 2003.
Zhang, R., Zhang, L., Zhang, Z., Wang, Y., Lu, M., Lapointe, M., and Chopp, M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol 50: 602-611. 2001.
27 - 국국국 문국 문문문 요요요 약요 약약약 –