저작자표시-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이 저작물을 영리 목적으로 이용할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Clinical Characteristics of recurred Oral Squamous
Cell Carcinoma
Munkhdul Altannamar
The Graduate School
Yonsei University
Department of Dentistry
Clinical Characteristics of recurred Oral Squamous
Cell Carcinoma
Directed by Professor Hyung Jun Kim
The Doctoral Dissertation
submitted to the Department of Dentistry
the Graduate School of Yonsei University
in partial fulfillment of the requirements for the degree of
Ph.D. in Dental Science
Munkhdul Altannamar
June 2016
ACKNOWLEDGEMENT
Foremost, I would like to express my sincere gratitude to my advisor Prof. Hyung Jun Kim for the continuous support of my Ph.D study and research, for his patience, motivation, enthusiasm, and immense knowledge. His guidance helped me in all the time of research and writing of this thesis.
Besides my advisor, I would like to thank the rest of my thesis committee: Prof. Choong Kook Yi, Prof. In Ho Cha, and Prof. Jong In Yook and Prof. Woong Nam for their encouragement, insightful comments. Also, very thankfully to Medical Mission Center of Yonsei University Health System that accepted me in scholarship of Avison International Fellowship program for financial supporting, and very appreciating for Dental College of Yonsei University scholarship program. And, many thanks to Dr. Yong Hoon Cha for helping me to
Last but not the least, I would like to thank my family: my mother, my wife and my daughters. Being with patience, strength and inspiration.
Table of Contents
List of figures ···ii
List of tables ··· iii
Abstract (English) ···iv
I. Introduction ··· 1
II. Materials and methods ··· 4
A. Patients ··· 4 B. Treatment ··· 4 C. Follow up ··· 5 D. Data Analysis··· 5 III. Results ··· 11 IV. Discussion ··· 25 V. Conclusion ··· 31 References ··· 33 Abstract (Korean) ··· 37
List of Figures
Figure 1. Clear resection margin, no evidence of microscopic
Figure 2. Close margin, histological evidence of carcinoma
Figure 3. Positive margin, evidence of microscopic carcinoma
Figure 4. lesion on margins, not invasive carcinoma
Figure 5. Perineural invasion
Figure 6. Vascular invasion
Figure 7.Kaplan-Meier time chased of recurrence curves
Figure 8.Cox regression risk factor (histologic grade)
List of Tables
Table 1. Gender and age
Table 2. Primary site
Table 3. Histologic grading
Table 4. TNM staging
Table 5. Size of tumor
Table 6. Nodal involvement
Table 7. Resection margin
Table 8. Perineural invasion
Table 9. Lymphovascular invasion
Table 10. Smoking status
Table 11. Drinking status
Table 12. Duration until recurrence
Table 13. Univariable analysis
ABSTRACT
Characteristics of recurred Oral Squamous Cell
Carcinoma
Munkhdul Altannamar, D.D.S.
Department of Dentistry
The Graduate School, Yonsei University
(Directed by Professor Hyung Jun Kim, D.D.S., M.S.D., Ph.D.)
Oral Squamous Cell Carcinoma (OSCC) is a common malignant tumor of the head and neck, and recurrence is an important prognostic factor in patients with OSCC, and takes around 85-90% of all Oral Cancers and may affect any anatomical site in the mouth. Oral Squamous Cell Carcinoma has a low survival rate, 34 to 66% five-year
survival after initial diagnosis, due to late diagnosis. Despite surgery, radiation and chemotherapy singularly or in combination, the fiveyear survival rate is poor, ultimately recurs in 25-48% of cases. Local and regional recurrences remain the most frequent recurrence types in patients with oral squamous cell carcinoma, and its incidence depends mainly on the site of the tumor, clinical stage and pathologic characteristics. The aim of this study was to analyze retrospectively all the oral squamous cell carcinoma cases, treated surgically in the department of Oral and Maxillofacial Surgery, Yonsei University, Seoul, Korea from 1991 until 2013 and clinicopathological characteristics that affect recurrence of oral squamous cell carcinoma. Total 308 patients with OSCC were found on medical records and selected 67 patients, 51 males and 16 females, mean age 56.7 years (range 19-76 years) diagnosed with recurrence of OSCC. Recurrence occurred at a median time of 1.8 years after the initial treatment, and more in male 51pts (16.6%), over the 60 (38%) year old than a female
16pts (5.2%). Status of nodal involvement, resection margin, perineural invasion and lymphovascular invasion were identified as factor influencing recurrence of OSCC. It is preventable, when may benefit from more radical surgery extended to selective or radical cervical lymph node dissection and more intensive surveillance during follow up and surgery, free flap application with postoperative radiotherapy and chemotherapy is the best option for improving the recurrence of the cancer and total survival times of patients.
Key words: Oral cancer, Oral Squamous cell carcinoma, Recurrence of Oral Squamous cell carcinoma, Head and neck cancer.
Characteristics of recurred Oral Squamous Cell
Carcinoma
Munkhdul Altannamar, D.D.S.
Department of Dentistry
The Graduate School, Yonsei University
(Directed by Professor Hyung Jun Kim, D.D.S., M.S.D., Ph.D.)
I. Introduction
Oral cancer is the 6th most common cancer and Oral squamous cell carcinoma (OSCC) has a remarkable incidence worldwide (Jerjes et al., 2010; Montebugnoli et al., 2014; Shah & Gil, 2009) and a fairly onerous prognosis, encouraging further research on factors that might modify disease outcome.
Most important risk factors for Oral Squamous Cell Carcinoma are use of tobacco and the regular drinking alcoholic beverages (Fang et al., 2013). However, infection with Human Papillomavirus (HPV) genotypes, and diet low in fresh fruits and vegetables have also recently been implicated in the aetiopathogenesis of Oral Squamous Cell Carcinoma (Shah & Gil, 2009). Approximately, which constitute 3-4% of all malignant tumors, are mostly carcinomas and 85 to 95% of all oral cancer is squamous cell carcinoma. The WHO expects a worldwide rising oral squamous cell carcinomas incidence in the next decades. In the last 30 years, the 5-year survival rate of patients with oral SCC has not improved despite advances in diagnostic techniques and improvements in treatment modalities. Indeed, the incidence and prevalence of oral SCC are increasing, particularly in younger persons (Feller & Lemmer, 2012). Despite great progress in chemotherapy, radiotherapy in the last three decades, the prognosis of the OSCC is poor due to aggressive local invasion and metastasis, leading to recurrence. Oral squamous cell carcinoma ultimately
recurs in 25–48% of cases (Koo, Lim, Lee, & Choi, 2006). Local and regional recurrences remain the most frequent recurrence types in patients with OSCC, and its incidence depends mainly on the site of the tumor, clinical stage and pathologic characteristics (Koo et al., 2006). In advanced head and neck cancers, several risk features result in a higher recurrence rate, including extracapsular extension of nodal diseases, microscopically involved margin, involvement of more than two nodes, vascular invasion, perineural infiltration,
and nodal involvement. Recurrence is an important prognostic
factor in patients with OSCC (Wang, Zhang, Yue, & Wang, 2013). Camisasca et al. have reported that 5yar survival rate was 92% in OSCC patients without recurrence and 30% in patients with recurrence (P < 0.001, long-rank test). Post-operative tumor recurrence leads to a poor prognosis and poor quality of life. Identifying factors that affect the recurrence of OSCC is important to reduce post-operative recurrence in clinical field.
II. Materials and methods
A. Patients
The total of 308 patients with OSCC were found on medical records and selected 67 patients with recurrence of OSCC, between in 1991-2013. All of them were patients at the Department of Oral and Maxillofacial Surgery, Yonsei University, Seoul, Korea. Clinical and Pathological data were reviewed from the medical records and histopathologically proven. Local recurrence, regional recurrence, or both were all defined as recurrence. Time to recurrence was determined by the duration from the first surgery to pathologically confirmed recurrence.
B. Treatment
The most of patients (94.11%) initially underwent curative surgery also surgery with postoperative radiotherapy (63%), surgery with chemotherapy (23.5%) and surgery with radiotherapy combined with chemotherapy (7.4%). Tumor
stage was determined according to the guidelines from the American Joint Committee on Cancer (2010) (Edge & Compton, 2010).
C. Follow up
Patients were followed up by hospital revisits, phone calls, or mails. The final date of follow-up was December 31, 2013. All patients were followed up for at least 5 years.
D. Data analysis
The initial step of this study was collected all data of clinical and histological characteristics of oral squamous cell carcinoma. The parameters were obtained from medical records and included age, gender, tumor location, clinical and pathologic stage, TNM, presence or absence of tumoral recurrence, treatment and tumor size. Tumor stage was defined in keeping with the clinical classification TNM (AJCC 2010) (Edge & Compton, 2010). Patients were divided into 2 groups with recurrence and non-recurrence. Local recurrence, regional
recurrence, or both were all defined as recurrence. Time to recurrence was determined by the duration from the first surgery to pathologically confirmed recurrence.
Histological diagnosis was performed according to recently published guidelines (Rosai, 2011), and all the cases were reviewed by the Department of Oral Pathology of Dental School, Yonsei University.
Pathological features included the following: Defined according to Kademani et al (Kademani et al., 2005).
Surgical margins: assessed at the closest point to the
tumor cells and classified in 4 categories according to the guidelines of the Royal College of Pathologists in the UK (Helliwell TR, 2005):
· Clear resection margin, no evidence of microscopic carcinoma (Figure1).
Figure1. Clear resection margin was diagnosed when the distance (arrow) between oral squamous cell carcinoma and the closest margin was more than 5 mm.
· Close margin, histological evidence of carcinoma (Figure2).
Figure2. Close margin, where the distance between oral squamous cell carcinoma and margin (arrow) is less than 5 mm.
· Positive margin, evidence of microscopic carcinoma (Figure3).
Figure3. Positive margin was considered involved when the distance between oral squamous cell carcinoma and margin was less than 1
mm.
· Epithelial precursor lesion on margins, not invasive carcinoma (Figure4).
Figure4. Epithelial precursor lesion on margins was diagnosed when marked architectural disturbance and cytological atypia were seen on the inked mucosa margin.
Infiltration depth: according to Ambrosch et al.
(Ambrosch, Kron, Fischer, & Brinck, 1995) as the maximum depth of tumor infiltration (millimeters) below the mucosal surface. In case of ulcerated or exophytic tumors, the reconstructed mucosal surface was used. Infiltration depth was measured on representative slides with the deepest infiltration. The cutoff was set to 4mm (Melchers et al., 2012).
Perineural invasion: pathologically as tumor invasion
of the perineural sheath and/or epineurium (Figure5).
Figure5. Perineural invasion (PNI) was diagnosed in all cases showing at least one peripheral nerve with evidence of neoplastic invasion.
Vascular invasion: as tumor emboli within vascular
structures (Figure6).
Figure6. Vascular invasion (arrow) (X100). Metastatic SCC in cervical neck node with vascular permeation
III. Results
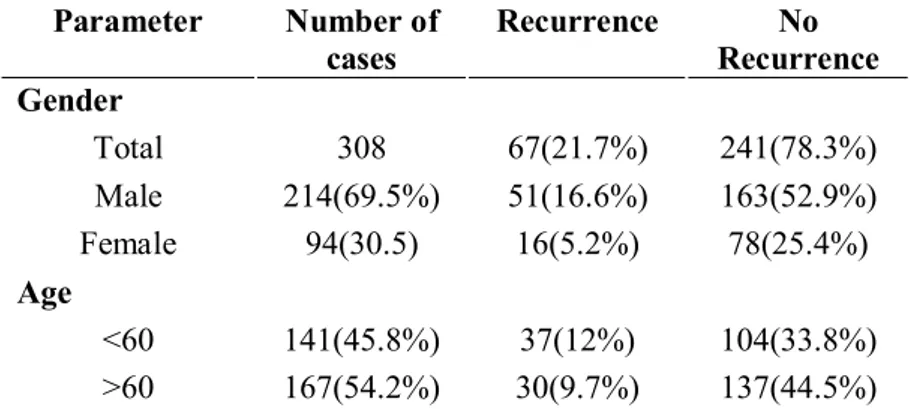
A total of 308 patients with OSCC were admitted between 1991 and 2013 to the Department of Oral and Maxillofacial Surgery, Yonsei University, Seoul, Korea. The 308 patients ranged from 19-76 years old, with a median age of 56.7 years. The gender ratio was 214 males, 94 females, approximately 2.3:1.
During follow up, were 67(21.7%) patients presented with recurrence, from them 51 males and 16 females, ranging in age from 19 to 76 years old (mean age 56.7 years) with a male predominance. Relevant patient demographics and clinicopathologic data are summarized in (Table1).
Table 1. Gender and age Parameter Number of cases Recurrence No Recurrence Gender Total 308 67(21.7%) 241(78.3%) Male 214(69.5%) 51(16.6%) 163(52.9%) Female 94(30.5) 16(5.2%) 78(25.4%) Age <60 141(45.8%) 37(12%) 104(33.8%) >60 167(54.2%) 30(9.7%) 137(44.5%)
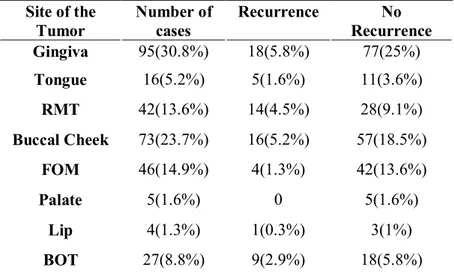
Gingiva 95(30.8%) and Buccal cheek 73(23.7%) cancer was the most common form of OSCC, followed by FOM 46(14.9%), RMT 42(13.6%), BOT 27(8.8%), Tongue 16(5.2%), Palate 5(1.6%) and Lip 4(1.3%) cancer. After selecting patients with recurrence, the site distribution shows that the most common of the cancer recurrence were the Gingiva (18/77 patients, 5.8%) and Buccal cheek (16/57 patients, 5.2%) followed by the RMT (14/28 patents, 4.5%), BOT (9/18 patients, 2.9%), Tongue (5/11 patients, 1.6%), FOM (4/42 patients, 1.3%) and Lip (1/3 patients, 0.3%) (Table2).
Table 2.Primary site Site of the Tumor Number of cases Recurrence No Recurrence Gingiva 95(30.8%) 18(5.8%) 77(25%) Tongue 16(5.2%) 5(1.6%) 11(3.6%) RMT 42(13.6%) 14(4.5%) 28(9.1%) Buccal Cheek 73(23.7%) 16(5.2%) 57(18.5%) FOM 46(14.9%) 4(1.3%) 42(13.6%) Palate 5(1.6%) 0 5(1.6%) Lip 4(1.3%) 1(0.3%) 3(1%) BOT 27(8.8%) 9(2.9%) 18(5.8%)
Histological diagnosis and grade of the tumors shows 60(24.4%) cases were histologically well differentiated, 151(61.4%) cases were moderately differentiated and 35(14.2%) cases were poorly differentiated of total 308 cases. From of them patients with moderately differentiation was the most common type 31(12.6%) cases recurred more than others, followed by poor differentiated 14(5.7%) cases and well differentiated 8(3.3%) cases (Table3).
Table 3. Histologic grading Differentiation Number of cases Recurrence No Recurrence Well 60(24.4%) 8(3.3%) 52(21.1%) Moderate 151(61.4%) 31(12.6%) 120(48.8%) Poor 35(14.2%) 14(5.7%) 21(8.5%)
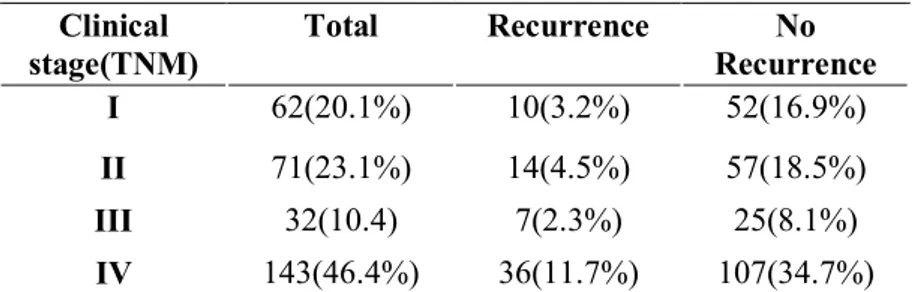
According to the 2010 AJCC(Edge & Compton, 2010) staging for OSCC shows 62(20.1%) cases were at stage I, 71(23.1%) at stage II, 32(10.4%) at stage III and 143(46.4%) at stage IV. From them 10(3.2%) cases at stage I, 14(4.5%) stage II, 7(2.3%) stage III, and with worst prognosis stage was IV with 36(11.7%) cases presented in recurred groups (Table4).
Table 4. TNM staging Clinical stage(TNM) Total Recurrence No Recurrence I 62(20.1%) 10(3.2%) 52(16.9%) II 71(23.1%) 14(4.5%) 57(18.5%) III 32(10.4) 7(2.3%) 25(8.1%) IV 143(46.4%) 36(11.7%) 107(34.7%)
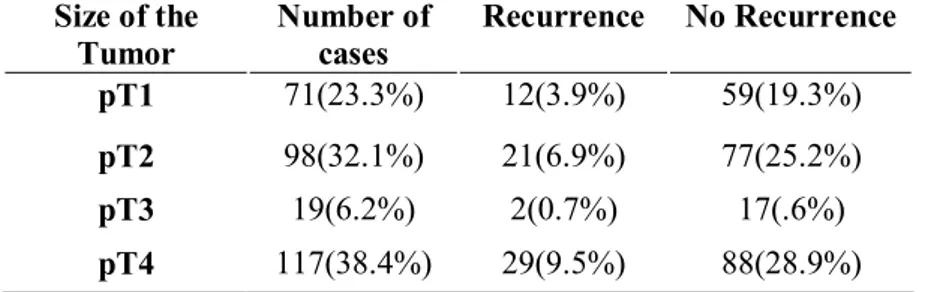
The size of the tumors in pT4 was most common 117(38.4%) cases, followed by pT2 98(32.1%) cases, pT1 71(23.3%) cases and pT3 19(6.2%) cases. By the way most common size was on recurred cases was pT2 and pT4 was over 50% bigger than pT1and pT3, 12(3.9%) cases in pT1, 21(6.9%) cases in pT2, 2(0.7%) cases in pT3, and 29(9.5%) cases in pT4, and pT4 shows, most of the patients diagnosed bigger than others respectively (Table5).
Table 5. Size of tumor
Size of the Tumor Number of cases Recurrence No Recurrence pT1 71(23.3%) 12(3.9%) 59(19.3%) pT2 98(32.1%) 21(6.9%) 77(25.2%) pT3 19(6.2%) 2(0.7%) 17(.6%) pT4 117(38.4%) 29(9.5%) 88(28.9%)
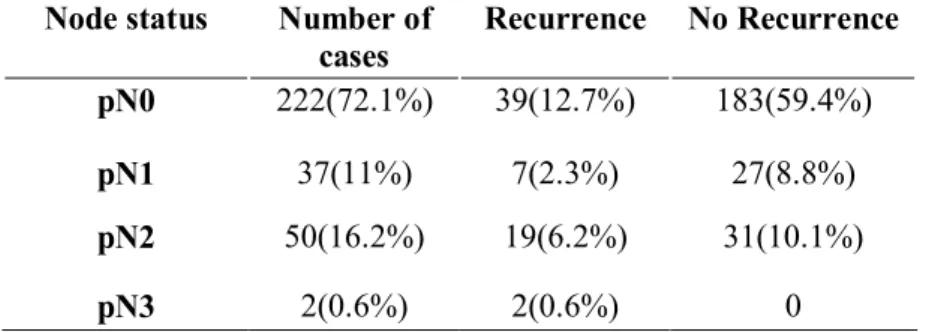
Nodal involvement was presented 222(72.1%) cases in pN0, 37(11%) cases in pN1, 50(16.2%) cases in pN2 and 2(0.6%) cases in pN3. From them, nodal involvement presented
in recurred patients 39(12.7%) cases in pN0, 7(2.3%) cases in pN1, 19(6.2%) cases in pN2, 2(0.6%) cases in pN3 (Table6).
Table 6. Nodal involvement
Node status Number of cases Recurrence No Recurrence pN0 222(72.1%) 39(12.7%) 183(59.4%) pN1 37(11%) 7(2.3%) 27(8.8%) pN2 50(16.2%) 19(6.2%) 31(10.1%) pN3 2(0.6%) 2(0.6%) 0
Also 172(80.8%) cases showed resection margin negative and 41(19.2%) cases positive. From them 39(18.3%) cases were within recurrence when resection margin is negative and 15(7%) cases with positive (Table7).
Table 7. Resection margin
Status Number of cases Recurrence No Recurrence Negative 172(80.8%) 39(18.3%) 133(62.4%) Close/Positive 41(19.2%) 15(7%) 26(12.2%)
Perineural invasion was presented in 53(72.6%) cases negative and 20(27.4%) cases positive. And in recurred patients shows 10(13.7%) cases negative and 9(12.3%) positive (Table8).
Table 8. Perineural invasion
Status Number of cases
Recurrence No Recurrence
Negative 53(72.6%) 10(13.7%) 43(58.9%)
Positive 20(27.4%) 9(12.3%) 11(15.1%)
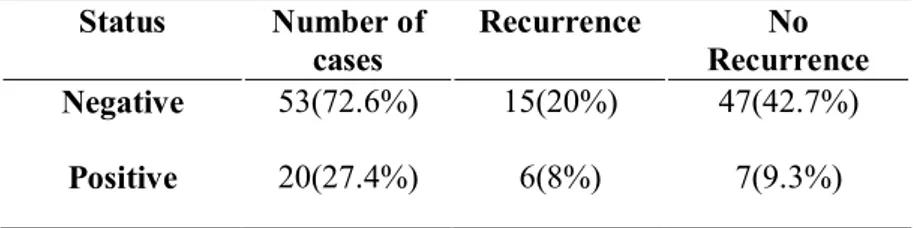
Lymphovascular invasion was presented 62(82.7%) cases in negative and 13(17.3%) cases in positive. And in patients with recurrence shows 15(20%) cases negative and 6(8%) positive (Table9).
Table 9. Lymphovascular invasion
Status Number of cases Recurrence No Recurrence Negative 53(72.6%) 15(20%) 47(42.7%) Positive 20(27.4%) 6(8%) 7(9.3%)
Smoking status shows in patients with recurrence was 38(12.4%) smokers more than 29(9.5%) non-smokers (Table10). Table 10. Smoking Status Number of cases Recurrence No Recurrence No 134(43.8%) 29(9.5%) 105(34.3%) Yes 172(56.2%) 38(12.4%) 134(43.8%)
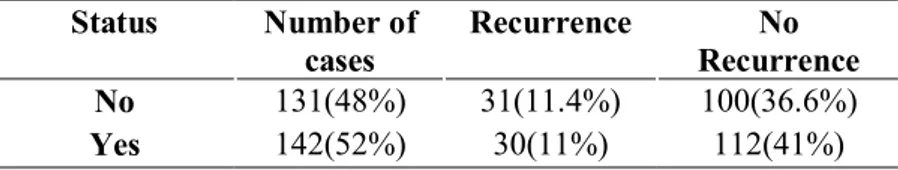
Drinking status presented 30(11%) patients consumed alcohol and 31(11.4%) patients no consumption of alcohol, shows almost same number (Table11).
Table 11. Drinking Status Number of cases Recurrence No Recurrence No 131(48%) 31(11.4%) 100(36.6%) Yes 142(52%) 30(11%) 112(41%)
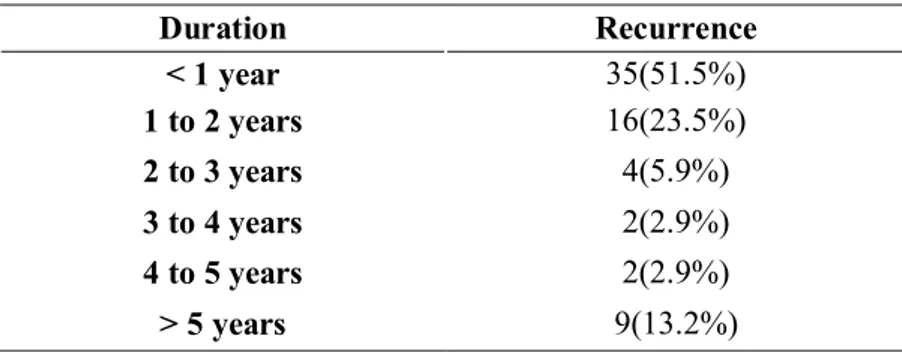
Total 67 patient presented recurrence or metastasis 35(52.2%) of them occurred within one year, 24(35.8%) until 5
years, and 8(11.9%) over 5 years after the initial treatment. More than 52.2% of recurrence occurs first year after treatment (Table12).
Table 12. Duration until recurrence
Duration Recurrence < 1 year 35(51.5%) 1 to 2 years 16(23.5%) 2 to 3 years 4(5.9%) 3 to 4 years 2(2.9%) 4 to 5 years 2(2.9%) > 5 years 9(13.2%)
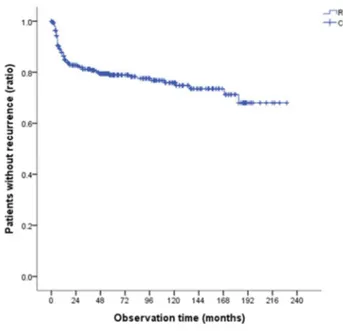
Kaplan-Meier time chased of recurrence curves demonstrating that recurrence rate of OSCC is 21.8% and most of recurrences occur within first 2 years after the first surgery, shows in figure 7.
Recurrence rate (K-M)
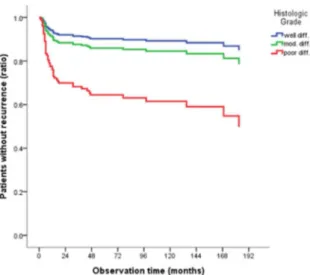
Risk factor
Figure 8. Cox regression risk factor demonstrates histologic grade
(p<0.002).
Figure 9. Cox proportional hazards regression risk factor
Statistical analysis was performed to determine the recurrence risk factors influencing recurrence of OSCC. In the univariate model shows, lymph node metastasis was associated with recurrence (p<0.000), whereas perineural invasion (p<0.003), lymphovascular infiltration (p<0.042), histologic grade (p<0.002) and resection margin (p<0.0029) were demonstrated statistically significant, associated with recurrence respectively (Table13).
Table 13. Univariable analysis
Multivariate analysis identified that histologic grade(p<0.036) and node status(p<0.03) are statistically significant and more important risk factors influencing OSCC recurrence (Table14).
Table 14. Multivariable analysis
IV. Discussion
SCC of the oral cavity has a deeply infiltrative nature as well as significant potential for occult neck metastasis (Koo et al., 2006). Tumor characteristics are responsible for the high recurrence rates following initial treatment of oral SCC (Nathanson et al., 1989; Pearlman, 1979). Factors that influence to the recurrence of OSCC have been extensively explored in recent years. Ebrahimi et al. have reported that T stage and N stage were important factors affecting regional recurrence in OSCC (Ebrahimi et al., 2011). Camisasca et al. have analyzed patients clinicopathologic data, including tumor sites, clinical and pathologic stage, histological grade, invasion mode, and perineural invasion (Camisasca et al., 2011). They have concluded that poor differentiation contributed to OSCC recurrence after surgery. Vazquez-Mahia et al. have reported that recurrence rate was 44.9% in patients with OSCC (Vazquez-Mahia et al., 2012), Jones reported a recurrence rate
of 41% (Jones, Lodge-Rigal, Reddick, Tudor, & Shockley, 1992), whereas Whitehurst reported a recurrence rate of 47% (Whitehurst & Droulias, 1977) with OSCC.
In our study, (1991–2013), Recurrence occurred 67 of 308 patients with OSCC (22.7%) and 23 of 67 were reoperated and 36(53.7%) were dead within 5 years after initial treatment, recurrence occurred at a median time of 1.8 years after the initial treatment which was lower than that reported 35.5% to 47.1% (Camisasca et al., 2011; Ebrahimi et al., 2011) in the studies by Camisasca, Ebrahimi, Vazquez-Mahia and Jones (Camisasca et al., 2011; Ebrahimi et al., 2011; Jones et al., 1992; Vazquez-Mahia et al., 2012). Therefor Koo B.S at al. also showed a similar result with 28% of the recurrence (Koo et al., 2006). The mean time of recurrence detection from the initial curative operation was 1 year. That mean 36(53.7%) of them occurred within 1st year and 16(23.8%) within 2nd years after the initial treatment. The 2-5 year survival rate was lower in patients with recurrence than in those without. Because most of the recurrences were within 2 years after the initial treatment,
close follow up of patients is important for the early detection of recurrence. At our hospital, patients are seen continuously and followed treatment. In our study, the rate of recurrence was higher in patients with an advanced pathologic stage, pathologic lymph node pN2-19(6.2%), pN3-2(0.6%) and positive resection margin 15(7%) compared to those with an early pathologic stage, negative lymph node or pN1-7(2.3%) and negative resection margin 39(18.3%). The lower recurrence rate in our study may be depends on following conditions: Pre-operative therapy, post-operative therapy, complete tumor resections. Tumor stage (p<0.256) and tumor size (p<0.253) are shows small possibility factors of recurrence of OSCC. Therefore, histologic grade (p<0.002) and node status (p<0.000) are most important risk factors that influencing recurrence of OSCC. Also, perineural invasion, lymphoscular infiltration and resection margin are significantly influencing risk factors.
Flap repair, chemotherapy, and radiotherapy may reduce the recurrences (Wang et al., 2013). Analyzing characteristics of recurred OSCC can help to establish or
facilitate the treatment standards and surgery remains the preferred treatment for it, and identifying relevant factors of tumor recurrence can help establish treatment standards. Surgery remains the preferred treatment for OSCC. However, for patients at T3-T4 stages and with poorly differentiated tumors, primary tumor resection margin should be expanded, 2 cm or more from the tumor, to ensure surgical safety. Flap repair should also be performed. Our results showed that the application of flap repair reduced local tumor recurrence. de Vicente et al. have followed up 98 patients with OSCC. They found that the mortality was 47.0% in patients with flap repair and was 67.3% in patients without flap repair (de Vicente, Rodriguez-Santamarta, Rosado, Pena, & de Villalain, 2012). The free flap repair application can improve the 5-year survival rate of patients. In addition, neck lymph nodes should be carefully cleaned while resecting the primary tumor. For patients with cN0 diseases, lymph nodes in the ipsilateral neck I-III regions should be selectively removed. Capote et al. have performed selective neck lymph node dissection on pT1N0M0
patients and primary tumor resection on patients with pT2N0M0 tumors. They found that the regional recurrence rate was significantly lower in patients who underwent selective neck lymph node dissection than in those who underwent primary tumor resection only. Thus, neck lymph node dissection is an important prognostic factor for the recurrence of OSCC (Capote et al., 2007). For neck lymph node-positive patients, radical neck dissection should be performed in the ipsilateral carotid I-V region. Because OSCC might migrate to the IIb region, the sternocleidomastoid may should be removed during surgery. Preoperative neoadjuvant chemotherapy and postoperative adjuvant chemotherapy or radiotherapy can also reduce recurrence and improve prognosis.
The patients in our study underwent preoperative chemotherapy and radiotherapy after surgery. The recurrence rate was 22.7 %, and the 5 year survival rate was 53,7%, both of which were satisfactory. Cooper at al. have also reported that postoperative radiotherapy and chemotherapy can improve disease-free survival and improve local and regional control rate
in patients with head and neck squamous cell carcinoma (Cooper et al., 2004). López Rodríguez et al. have reported that preoperative radiotherapy and chemotherapy for head and neck squamous cell carcinoma at N2-N3 stage can completely control neck lymph node metastasis and achieve local and regional effectiveness (Lopez Rodriguez, Cerezo Padellano, Martin Martin, & Counago Lorenzo, 2008).
OSCC of the head and neck is commonly associated with the use of alcohol and tobacco. Smoking and alcohol consumption to be major risk factors for OSCC. Almost half of our patients were tobacco smokers and alcohol drinkers.
In this study, we analyzed clinical and pathological characteristics of recurred OSCC and discussed some perspectives for clinical reference.
V. Conclusion
In conclusion, in this study 67 of 308 patients surgically treated for recurred OSCC, recurrences and/or metastases were found in 22.7% of patients, mostly at the primary site within 1.8 years after the initial treatment.
Although half of patients were Stage IV, recurrence rate was relatively lower than previous reports.
It implies that serial surgical procedures such as wide excision, neck dissection and reconstruction with free flap for treating advanced OSCC has benefit in removing malignant lesions clearly.
Pathologic features are more important than population based features in locoregional recurrence of OSCC.
Among clinical features, only TNM stage and primary tumor sites show a little possibility in recurrence of OSCC.
Node status is an important risk factor in predicting prognosis both clinically and pathologically.
LVI, PNI, Histologic grade and resection margin status are critical risk factors while almost of them are biologically related with cellular invasion and migration, so called epithelial mesenchymal transition (EMT).
Missing values in several pathologic features still remain further study and close communication between surgeon and pathologist.
References
Ambrosch, P., Kron, M., Fischer, G., & Brinck, U. (1995). Micrometastases in carcinoma of the upper
aerodigestive tract: detection, risk of metastasizing, and prognostic value of depth of invasion. Head Neck,
17(6), 473-479.
Camisasca, D. R., Silami, M. A., Honorato, J., Dias, F. L., de Faria, P. A., & Lourenco Sde, Q. (2011). Oral
squamous cell carcinoma: clinicopathological features in patients with and without recurrence. ORL J
Otorhinolaryngol Relat Spec, 73(3), 170-176. doi:
10.1159/000328340
Capote, A., Escorial, V., Munoz-Guerra, M. F., Rodriguez-Campo, F. J., Gamallo, C., & Naval, L. (2007). Elective neck dissection in early-stage oral squamous cell carcinoma--does it influence recurrence and survival? Head Neck, 29(1), 3-11. doi:
10.1002/hed.20482
Cooper, J. S., Pajak, T. F., Forastiere, A. A., Jacobs, J.,
Campbell, B. H., Saxman, S. B., . . . Radiation Therapy Oncology Group, I. (2004). Postoperative concurrent radiotherapy and chemotherapy for high-risk
squamous-cell carcinoma of the head and neck. N Engl
J Med, 350(19), 1937-1944. doi:
10.1056/NEJMoa032646
de Vicente, J. C., Rodriguez-Santamarta, T., Rosado, P., Pena, I., & de Villalain, L. (2012). Survival after free flap reconstruction in patients with advanced oral squamous cell carcinoma. J Oral Maxillofac Surg, 70(2), 453-459. doi: 10.1016/j.joms.2011.02.020
Ebrahimi, A., Clark, J. R., Zhang, W. J., Elliott, M. S., Gao, K., Milross, C. G., & Shannon, K. F. (2011). Lymph node ratio as an independent prognostic factor in oral
squamous cell carcinoma. Head Neck, 33(9), 1245-1251. doi: 10.1002/hed.21600
Edge, S. B., & Compton, C. C. (2010). The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann
Surg Oncol, 17(6), 1471-1474. doi:
10.1245/s10434-010-0985-4
Fang, Q. G., Shi, S., Li, Z. N., Zhang, X., Liua, F. Y., Xu, Z. F., & Sun, C. F. (2013). Squamous cell carcinoma of the buccal mucosa: Analysis of clinical presentation, outcome and prognostic factors. Mol Clin Oncol, 1(3), 531-534. doi: 10.3892/mco.2013.86
Feller, L., & Lemmer, J. (2012). Oral Squamous Cell
Carcinoma: Epidemiology, Clinical Presentation and Treatment. Journal of Cancer Therapy, 03(04), 263-268. doi: 10.4236/jct.2012.34037
Helliwell TR, W. J. (2005). Datasets for Histopatholgy
Reports on Head and Neck Carcinoma and Salivary Neoplasms.
Jerjes, W., Upile, T., Petrie, A., Riskalla, A., Hamdoon, Z., Vourvachis, M., . . . Hopper, C. (2010).
Clinicopathological parameters, recurrence,
locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol,
2, 9. doi: 10.1186/1758-3284-2-9
Jones, K. R., Lodge-Rigal, R. D., Reddick, R. L., Tudor, G. E., & Shockley, W. W. (1992). Prognostic factors in the recurrence of stage I and II squamous cell cancer of the oral cavity. Arch Otolaryngol Head Neck Surg, 118(5), 483-485.
Kademani, D., Bell, R. B., Bagheri, S., Holmgren, E., Dierks, E., Potter, B., & Homer, L. (2005). Prognostic factors in intraoral squamous cell carcinoma: the influence of histologic grade. J Oral Maxillofac Surg, 63(11), 1599-1605. doi: 10.1016/j.joms.2005.07.011
Koo, B. S., Lim, Y. C., Lee, J. S., & Choi, E. C. (2006). Recurrence and salvage treatment of squamous cell
carcinoma of the oral cavity. Oral Oncol, 42(8), 789-794. doi: 10.1016/j.oraloncology.2005.11.016
Lopez Rodriguez, M., Cerezo Padellano, L., Martin Martin, M., & Counago Lorenzo, F. (2008). Neck dissection after radiochemotherapy in patients with locoregionally advanced head and neck cancer. Clin Transl Oncol,
10(12), 812-816.
Melchers, L. J., Schuuring, E., van Dijk, B. A., de Bock, G. H., Witjes, M. J., van der Laan, B. F., . . . Roodenburg, J. L. (2012). Tumour infiltration depth >/=4 mm is an indication for an elective neck dissection in pT1cN0 oral squamous cell carcinoma. Oral Oncol, 48(4), 337-342. doi: 10.1016/j.oraloncology.2011.11.007
Montebugnoli, L., Gissi, D. B., Flamminio, F., Gentile, L., Dallera, V., Leonardi, E., . . . Foschini, M. P. (2014). Clinicopathologic parameters related to recurrence and locoregional metastasis in 180 oral squamous cell carcinomas. Int J Surg Pathol, 22(1), 55-62. doi: 10.1177/1066896913511982
Nathanson, A., Agren, K., Biorklund, A., Lind, M. G., Andreason, L., Anniko, M., . . . et al. (1989). Evaluation of some prognostic factors in small squamous cell carcinoma of the mobile tongue: a multicenter study in Sweden. Head Neck, 11(5), 387-392.
Pearlman, N. W. (1979). Treatment outcome in recurrent head and neck cancer. Arch Surg, 114(1), 39-42.
Rosai, J. (2011). Standardized surgical pathology reporting
for major tumor types. Oral cavity and oropharynx carcinoma. In: Rosai J, ed. Rosai and Ackerman’s Surgical Pathology (10th ed. ed.). St Louis: Mosby
Elsevier.
Shah, J. P., & Gil, Z. (2009). Current concepts in management of oral cancer--surgery. Oral Oncol, 45(4-5), 394-401. doi: 10.1016/j.oraloncology.2008.05.017
Vazquez-Mahia, I., Seoane, J., Varela-Centelles, P., Tomas, I., Alvarez Garcia, A., & Lopez Cedrun, J. L. (2012).
Predictors for tumor recurrence after primary definitive surgery for oral cancer. J Oral Maxillofac Surg, 70(7), 1724-1732. doi: 10.1016/j.joms.2011.06.228
Wang, B., Zhang, S., Yue, K., & Wang, X. D. (2013). The recurrence and survival of oral squamous cell carcinoma: a report of 275 cases. Chin J Cancer,
32(11), 614-618. doi: 10.5732/cjc.012.10219
Whitehurst, J. O., & Droulias, C. A. (1977). Surgical treatment of squamous cell carcinoma of the oral tongue: factors influencing survival. Arch Otolaryngol, 103(4), 212-215.
ABSTRACT (IN KOREAN)
재발된 구강편평세포암종의 특징
<지도교수 김 형 준> 연세대학교 대학원 치의학과 뭉 크 돌 구강편평세포암은 두경부 영역에서 가장 흔한 악성 종양이며 재발은 환자의 생존과 밀접한 관련이 있다. 구강편평세포암의 5 년 생존율은 34~66% 정도로 비교적 낮은 편에 속하며 이것은 뒤늦은 진단에서 기인한다. 수술 요법 외에 항암 또는 방사선 치료가 단독 혹은 복합으로 시행되더라도 5 년 생존율은 여전히 좋지 않으며 약 25~48% 정도의 재발율을 보이고 있다. 국소적 재발은 구강편평세포암 환자에게서 나타나는 재발의 대부분을 차지하며 종양의 해부학적 발생 위치, 임상 병기, 병리적 특성에 의존한다. 본 연구는 연세대학교 치과대학병원 구강악안면외과에서 1991 년부터 2013 년까지 외과적으로 치료받았던 구강편평세포암 환자를 후향적으로 분석하여 재발에 영향을 주는 요소의 임상병리적 특성을 파악하고자 한다. 총 308 명의 구강편평세포암 환자가 의무기록에 근거하여 수집되었고 그 중 67 명, 남자 51 명 여자 16 명이 재발 된 것으로 나타났으며 평균 연령은 56.7 세(19-76 세)였다. 재발이 나타나는 중위 기간은 최초 치료 이후 1.8 년 이었으며 남성과 고령 환자에서 더 많이 재발하는 것으로 관찰되었다. 림프절 전이, 안전 절제연 확보, 신경침범 및 림프혈관 침범은 구강편평세포 재발에 유의미한 영향을 주는 요소로 분석되었다. 이상의 결과를 토대로 구강편평세포암의 재발은 선택적 혹은 근치적 경부 곽청술을 포함한 광범위 절제술과 유리피판을 이용한 재건, 적절한 술후 방사선 및 화학치료, 주의 깊은 술후 감시에 의해 예방되어 질 수 있을 것으로 사료된다. 핵심어 : 구강암, 구강편평세포암, 구강편평세포암 재발, 두경부암, 구인두