저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
1
Doctoral Thesis in Medicine
Prognostic significance of
microvascular invasion
and related microRNAs
after hepatic resection for
hepatocellular carcinoma
Graduate School of Ajou University
Department of Medicine
2
Prognostic significance of microvascular
invasion and related microRNAs after
hepatic resection for hepatocellular
carcinoma
지도교수 왕희정
이 논문을 의학 박사학위 논문으로 제출함.
2018년 8월
아 주 대 학 교 대 학 원
의 학 과
박용근
3
박용근의 의학 박사학위 논문을 인준함.
심 사 위 원 장
김 진 홍
인
심 사 위 원
왕 희 정
인
심 사 위 원
정 재 연
인
심 사 위 원
정 철 운
인
심 사 위 원
정 운 용
인
아 주 대 학 교 대 학 원
2018년 7월 6일
4
ACKNOWLEDGEMENTS
Firstly, I would like to thank my advisor Professor Wang, Hee-Jung for the continuous
support and encouragement during my PhD studies. An enormous thanks also goes to my
fellow surgical trainees and professors in the period of my surgical residency and
fellowship.
I specially thanks to my parents Park, Kwangsoo and Yoo, Soonyea. I want to express my
deepest gratitude to them for their commitment to the education of their children, me and
my brother, Dr. Park, Seung-Keun. Words cannot express how much I appreciate my
parents for all of the love that I have received from them.
i
ABSTRACT
Prognostic significance of microvascular invasion in tumor stage for hepatocellular carcinoma
Background: The presence of microvascular invasion (McVI) in hepatocellular carcinoma (HCC) has been proposed as a cause of recurrence and poor survival, although this has not been officially emphasized in staging systems. Thus, we conducted a retrospective study to investigate the prognostic importance of McVI in tumor staging in patients with HCC who underwent hepatic resection. Patients and Methods: A retrospective analysis was performed of patients who underwent hepatic resection for HCC at our center from 1994 to 2012. Patients with HCC were classified into four groups based on the presence of McVI and extent of gross vascular invasion (VI). Results: The 5-years overall and recurrence-free survival rates of 676 patients were 63.3% and 42.6%, respectively. There was no difference in tumor recurrence or survival rate between patients with HCC and McVI without gross VI and those with gross VI confined to segmental/sectional branches. Multivariate analysis revealed that the extent of VI based on the presence of McVI and gross VI was independently associated with tumor recurrence and overall survival. Conclusions: McVI was revealed to be an important risk factor similar to gross VI confined to a
segmental/sectional branch in patients with HCC who underwent hepatic resection. This finding should be considered when estimating the stage for prognosis.
Conditional survival analysis demonstrates that recurrence risk of surgically treated hepatocellular carcinoma evolves with time
Objective: The study aim was to investigate long-term change in tumor recurrence risk in patients with hepatocellular carcinoma (HCC) after hepatic resection. Recurrence probability over time was estimated by conditional survival (CS) analysis. Patients and Methods: Early-stage HCC patients with hepatic resection were selected for inclusion from our surgery database. Variables predictive of tumor recurrence were identified by univariate and multivariate analyses. Five-year recurrence-free CS probability was calculated for all patients and for risk groups stratified by independent
predictors. Results: In this series of 436 patients, tumor size >5 cm, microvascular invasion, positive resection margin, liver cirrhosis, and a indocyanine green retention ratio at 15 minutes
ii
(ICG-R15) >20% were independently predictive of tumor recurrence. The estimated 5-year recurrence-free CS probability improved with each additional year of recurrence-free survival, and the improvement was significantly greater in the high risk than in the low or intermediate risk groups. Conclusion: CS provides added value during follow-up of early-stage HCC patients treated by surgical resection.
MicroRNA-9 overexpression is associated with microvascular invasion and poor survival after hepatic resection for hepatocellular carcinoma
Purpose: Although microvascular invasion (McVI) has prognostic value for patients with hepatocellular carcinoma (HCC) who have undergone hepatic resection, few studies have investigated the relationship between McVI and the aberrant expression of microRNAs. This study identified microRNAs selectively expressed in HCC with McVI and investigated their prognostic roles. Patients and methods: Clinical data and microRNA expression profiles for 355 HCC patients were extracted from The Cancer Genome Atlas database. MicroRNAs that were differentially expressed in the patients with McVI and those without vascular invasion were identified and investigated as potential prognostic factors for HCC. Results: MicroRNA-9-5p was upregulated more (fold change [FC] 2.30; false discovery rate [FDR] < 0.001) and microRNA-675-5p was downregulated more (FC 0.52; FDR = 0.005) in the patients with McVI. Multivariate analysis revealed that the types of surgery (HR 1.718, 95% CI 1.169–2.524, P = 0.006), the presence of a residual tumor (HR 3.475, 95% CI 1.507–8.013, P = 0.003) and advanced TNM stage (HR 1.817, 95% CI 1.163–2.839, P = 0.009) were independently associated with tumor recurrence, and that advanced TNM stage and overexpression of microRNA-9 were independent risk factors for overall survival after hepatic resection for HCC (HR 1.671, 95% CI 1.114–2.508, P = 0.013 and HR 3.451, 95% CI 1.796–6.630, P < 0.001, respectively). Conclusion: Overexpression of microRNA-9-5p was associated with McVI and poor survival of patients after hepatic resection for HCC.
iii
Table of Contents
1) List of Text
I. Introduction ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 1
II. Prognostic significance of microvascular invasion in
tumor stage for hepatocellular carcinoma ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 3
III. Conditional survival analysis demonstrates that
recurrence risk of surgically treated hepatocellular
carcinoma evolves with time ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 27
IV. MicroRNA-9 overexpression is associated with
microvascular invasion and poor survival after hepatic
resection for hepatocellular carcinoma: an analysis using
The Cancer Genome Atlas database ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 45
V. Appendix
A. Bibliography ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧
64
iv
2) List of Figures
II. Prognostic significance of microvascular invasion in tumor stage for hepatocellular carcinoma
Figure 1. Flow diagram shows the selection of patients who were eligible for this study. ‧‧‧‧‧‧‧‧‧‧‧‧‧ 7 Figure 2. Comparison of (a) recurrence-free and (b) overall survival of patients stratified into groups A–D. ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 14
Figure 3. Summary of statistically significant clinicopathological factors on (a) recurrence-free survival and (b) overall survival using the Cox regression proportional hazards model. ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 22
III. Conditional survival analysis demonstrates that recurrence risk of surgically treated hepatocellular carcinoma evolves with time
Figure 4. Flow diagram showing the selection of patients who were eligible for this study. ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 31 Figure 5. Cumulative recurrence-free and overall survival of the 436 patients with resected early-stage HCC estimated by the Kaplan–Meier method. ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 33
Figure 6. Five-year recurrence-free conditional survival (CS) at each additional year after hepatic resection. ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 35
Figure 7. Recurrence-free survival curves (a) and five-year recurrence-free CS probability (b) in three risk groups. ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 38
Figure 8. Five-year recurrence-free CS probability with (a) tumor size less than or greater than 5 cm, (b) ICG-R15 values less than or more than 20% (c) presence or absence of microvascular invasion, and (d) presence or absence of background liver cirrhosis. ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 40
IV. MicroRNA-9 overexpression is associated with microvascular invasion and poor survival after hepatic resection for hepatocellular carcinoma: an analysis using The Cancer Genome Atlas database
v
Figure 9. Study flowchart. McVI, microvascular invasion; HCC, hepatocellular carcinoma; DESeq., differentially expressed sequences ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 47
Figure 10. Comparison of (a) recurrence-free survival and (b) overall survival of patients between patients with microvascular invasion [McVI (+), Group B] and without vascular invasion [McVI (−), Group A].; NS, not significant ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 52
Figure 2. Comparison of (a) recurrence-free survival and (b) overall survival curves between over and under microRNA-9 expression ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 59
vi
3) List of Tables
II. Prognostic significance of microvascular invasion in tumor stage for hepatocellular carcinoma
Table 1. Comparison of clinicopathological data for patients classified into four groups. ‧‧‧‧‧‧‧‧‧‧‧‧‧ 9 Table 2. Univariate analysis of factors predictive of recurrence-free and overall survival. ‧‧‧‧‧‧‧‧‧ 16
III. Conditional survival analysis demonstrates that recurrence risk of surgically treated hepatocellular carcinoma evolves with time
Table 3. Univariate and multivariate analysis to identify prognostic factors associated with tumor recurrence. ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 36
IV. MicroRNA-9 overexpression is associated with microvascular invasion and poor survival after hepatic resection for hepatocellular carcinoma: an analysis using The Cancer Genome Atlas database
Table 4. Comparison of clinicopathological data of hepatocellular carcinoma patients with no vascular invasion (Group A) or with microvascular invasion (Group B) ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 50
Table 5. Univariate analysis of factors predictive of recurrence-free survival and overall survival ‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧‧ 55
1
I. Introduction
Hepatocellular carcinoma (HCC) is a frequently diagnosed cancer and a leading cause of cancer-related deaths worldwide. [1] Epidemiological evidence confirms that both HCC incidence and mortality increased between 2000 and 2010. [2] Surveillance of high-risk groups helps in the early detection of HCC, thus making curative treatment more likely. Hepatic resection is a potentially curative treatment of early-stage HCC in patients with preserved liver function. [3] With considerable improvements in the selection process and intra- and preoperative care, mortality rates have significantly decreased, [4] but the high rate of tumor recurrence is a major shortcoming and the main cause of poor outcomes. [5,6]
Studies evaluating patients stratified by various predictors of recurrence risk [4,7] have identified microvascular invasion (McVI) as a factor that can affect the prognosis of postoperative recurrence. Subsequent studies have focused on preoperative prediction of McVI. [8,9] When HCC tumor progresses, it may invade neighboring vessels. [10] Gross vascular invasion by tumor cells is a well-recognized negative prognostic feature of HCC, which has been reflected in official staging systems. [11-13] However, it remains unclear how much McVI provides prognostic information for patients with HCC from the viewpoint of the extent of tumor invasion or extension. Official staging systems, such as the Liver Cancer Study Group of Japan tumor nodes metastasis, and the Barcelona Clinic for Liver Cancer staging systems, contain no mention of McVI. Considered as the first step of metastatic dissemination via the vascular route, prognostic impact of McVI may be intuitively thought to be placed between non-VI and gross invasion of vessels. However, there is no strong evidence to support this speculation. So, we aimed to clarify the importance of McVI as the degree of local tumor invasion or extension in tumor stage for HCC.
Although McVI has prognostic value for clinicians and patients in the immediate postoperative period, there is little evidence that they are useful to forecast the probability of recurrence after a long tumor-free period. The reason is that the values of parameters evaluated in previous studies for prediction of recurrence were obtained around the time of surgery, and thus, could not provide information about changes in postoperative oncologic risks. We generally assume that risk of recurrence would decrease. Patients at high risk of tumor recurrence who remain recurrence-free for long periods may not have the same risk as that at the time of surgery. Unfortunately, the supporting evidence of this is weak. None of the previous studies have accounted
2
for the effect of recurrence-free survivorship on the evolving risk of tumor recurrence in HCC. Different prediction tools or methods may be needed to provide an insight into change in prognosis over time. This need has been addressed recently using the concept of conditional survival (CS) analysis. [14,15] We analyzed recurrence-free CS in patients with hepatic resection for early-stage HCC, and assessed independent risk factors of tumor recurrence (including McVI) at initial presentation and changes in their predictive powers over a long recurrence-free period.
Vascular invasion of HCC tumor is considered to be a reflection of aggressiveness. [16] However, little information is available regarding this tumor progression mechanism, which remains to be elucidated. A possible postulation is that portal vein or hepatic vein tumor invasion may simply be an effect of tumor topography, which means that this aggressive phenomenon may happen only because of the close anatomical proximity to neighboring vessels. However, mounting evidence has demonstrated that epithelial–mesenchymal transition (EMT) participates in the aggressive phenotypical changes in cancer cells, lead to their invasion of adjacent tissues. [17,18] In addition, comprehensive genomic data analysis has facilitated improved overall understanding of tumor biology. [19] Recently, several types of small non-coding RNA have been shown to play critical roles in tumorigenesis and tumor progression; some of them regulate EMT directly or indirectly, which are consequently involved in tumor invasion or metastasis. [20,21] MicroRNAs are a class of endogenously expressed small, non-coding RNAs. Recent studies have identified clinically significant abnormal patterns of microRNA expression in HCC, with some showing a marked association with aggressive tumor phenotypes or poor survival. [22] However, few studies have investigated the relationship between McVI and aberrant microRNA expression. Therefore, we also aimed to elucidate microRNAs that act as important contributors to the McVI of HCC, using The Cancer Genome Atlas (TCGA) database.
3
II. Prognostic significance of microvascular invasion in
tumor stage for hepatocellular carcinoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most commonly diagnosed cancers and is responsible for a high incidence of cancer-related deaths throughout the world. [1] However, treatment with curative intention, such as hepatic resection, liver transplantation (LT), and locoregional therapies, can only be applied in approximately 30% of patients with early-stage HCC. [2] Although these therapeutic modalities have improved the overall survival (OS), long-term outcomes remain poor because of high rates of tumor recurrence. Vascular invasion (VI) is a key contributor to tumor recurrence, which leads to dismal outcomes in patients with HCC. [3]
When HCC tumor progresses, it may invade neighboring vessels. [4] VI by tumor cells is a well-recognized negative prognostic feature of HCC, which has been reflected in official staging systems. [5-7] In the tumor nodes metastasis (TNM) stage based on the criteria of the Liver Cancer Study Group of Japan (LCSGJ), VI is one of three factors for determining the T stage with tumor size and numbers. [6] According to the Barcelona Clinic for Liver Cancer (BCLC) staging, HCC with gross VI is classified as advanced stage, which most likely will not benefit from curative treatment. [7] However, it remains unclear how much microvascular invasion (McVI) provides prognostic information for patients with HCC from the viewpoint of the extent of tumor invasion or extension.
Studies evaluating patients stratified by various predictors of recurrence risk have identified McVI as a factor that can affect the prognosis of postoperative recurrence. [8,9] McVI is also reported in several studies to be an important risk factor for HCC recurrence after LT. [10,11] Subsequent studies have focused on preoperative prediction of McVI to aid the decision-making process for optimal treatment option in patients with HCC. [12,13]
Considered as the first step of metastatic dissemination via the vascular route, prognostic impact of McVI may be intuitively thought to be placed between non-VI and gross invasion of
4
vessels. However, there is no strong evidence to support this speculation. The protocol developed by the College of American Pathologists considers McVI the same as gross VI confined to segmental/sectional branches of HCC on the current American Joint Committee on Cancer (AJCC)/International Union for Cancer Control (UICC) tumor TNM staging system. [14] Unfortunately, there is no mention of the prognostic significance of McVI on other staging systems, such as LCSGJ TNM, or BCLC. [6,7] In this retrospective study, we aimed to clarify the importance of McVI as the degree of local tumor invasion or extension in tumor stage for HCC.
PATIENTS and METHODS
A retrospective analysis was performed on a database of patients who underwent surgical procedures for HCC at our center between September 1994 and December 2012. Data were extracted from prospectively collected database records, which included demographics, etiology of underlying liver disease, pathological findings of the specimen, surgical results, and oncological outcomes. Patients lost during follow-up were censored.
We preferentially considered and attempted surgical resection for all patients newly diagnosed with HCC in the Department of Surgery and all referred patients from the Department of Gastroenterology and other institutions if liver function was preserved and the state of HCC was not technically inoperable. Liver function was assessed by the Child-Turcotte-Pugh (CTP) classification and indocyanine green retention rate at 15 min (ICG-R15) value. For many years, our approach to determine the extent of resection has been based on a prediction scoring system. [15] We did not abandon hepatic resection because of the existence or extent of gross VI. Major hepatic resection was defined as the removal of three or more segments according to the Brisbane classification. [16] Intraoperative ultrasound was routinely used to detect any additional nodules and to aid in the determination of the most optimal resection plane.
Tumors were staged based on postoperative pathological findings according to the AJCC/UICC TNM and LCSGJ staging system. [5,6] McVI was defined by tumor within a vascular space lined by endothelium, identified only on microscopy in the capsule or noncapsular fibrous septa, or liver tissue surrounding the tumor. [17] In all cases, tumor grade was defined by the poorest degree of differentiation using the Edmondson–Steiner grades, identified within the tumor
5
upon pathological analysis of the entire specimen. [18] Portal vein tumor thrombus (PVTT) and hepatic vein tumor thrombus (HVTT) was classified into five and four groups respectively according to the General Rules for the Study of Primary Liver Cancer by the Korean Liver Cancer Study Group. [19]
Follow-up investigations consisted of imaging studies with serum α-fetoprotein (AFP) level. Biochemical liver function tests, AFP level, and abdominal computed tomography (CT) scan were conducted every 3 months after discharge during the first 2 years and approximately every 3–6 months for the following years. Tumor recurrence was diagnosed by the combination of elevated tumor markers and consistent radiological findings. If recurrence was highly suspected without clear evidence on an imaging study, hepatic arteriography and lipiodol CT scans were performed. Patients with tumor recurrence were managed with various therapeutic modalities, including local ablation, re-resection, and salvage LT. Patients with multiple or large tumors and/or hepatic dysfunction underwent transcatheter arterial chemoembolization (TACE). Targeted therapy with sorafenib and radiation therapy were also adopted for advanced or metastatic tumors.
Statistical analysis
Variables preoperatively and pathologically stratified were analyzed using univariate and multivariate analyses to determine independent predictors of oncological outcome. All continuous variables were expressed as mean ± standard deviation or median (minimum–maximum range). The optimal cutoff values for continuous variables for use in the Kaplan–Meier survival analyses were estimated by receiver operating characteristic (ROC) curve analysis. Survival rates and curves were estimated using the Kaplan–Meier method and compared using the log rank test. Multivariate analysis was performed using the Cox regression proportional hazards model to identify independent factors that determined recurrence-free survival (RFS) and OS. All statistical analyses were performed using R-packages, version 3.3.1. [20] All P values <0.05 were considered statistically significant.
RESULTS
6
Patients who underwent primary hepatic resection were eligible for the study. Exclusion criteria were patients who underwent primary LT for HCC (n = 93) and those undergoing reoperation (n = 79), such as repeated hepatic resection, salvage or repeated LT, and hepatic resection following LT. The diagnosis of HCC was confirmed by pathological examination in all cases. A total of 33 patients who had combined HCC and cholangiocarcinoma and three with distant metastasis at the operation time were also excluded. This retrospective study was performed on the remaining 676 patients (Figure 1), and their clinicopathological details are summarized in Table 1. There were 530 male (78.4%) and 146 female (21.6%) patients (median age, 52 years; range, 20–76 years). Among the patients, 516 (78.5%) tested positive for serum hepatitis B surface antigen and 38 (6%) tested positive for hepatitis C antibody; 36 (5.3%) and 14 (2.1%) patients had CTP classes B and C, respectively, and 623 (92.6%) had class A disease. Serum AFP level was normal in 197 patients (29.6%), abnormal but less than 400 ng/mL in 251 (37.7%), and more than 1000 ng/mL in 217 (32.7%). 232 (40.0%) patients underwent preoperative TACE. Among the operations, 262 (38.7%; approximately 4/10 rate) were major hepatic resections, whereas 184 (27.2%) were segmentectomies or bisegmentectomies and 230 (34.1 %) were minor resections.
7
Figure 3. Flow diagram shows the selection of patients who were eligible for this study. CCC, cholangiocarcinoma.
8
Overall median follow-up period was 40 (1–204) months. The 90-day mortality rate after hepatic resection because of post-hepatectomy liver failure or sepsis was 1.9% (13 of 676). During the follow-up period, 55.1% (365 of 663) of the patients had tumor recurrence and 35.6% (236 of 663) died. The 5-year OS and RFS rates were 63.3% and 42.6%, respectively.
Pathological analysis postoperatively revealed that 328 patients (48.5%) had combined McVI: 193 had McVI without and 135 had McVI with gross VI. According to the extent of PVTT, 537 patients (79.4%) had Vp0, 58 (8.6%) had Vp1, 41 (6.1%) had Vp2, 22 (3.3%) had Vp3, and 18 (2.7%) had Vp4. A total of 29 patients (4.3%) had tumor with hepatic vein invasion. Based on the extent of HVTT, the patients were classified into four groups: Vv0 (n = 647), Vv1 (n = 19), Vv2 (n = 5), and Vv3 (n = 5). The patients were also divided into four groups based on the existence of McVI and extent of gross VI: group A, no McVI or gross VI; group B, McVI without gross VI; group C, VI confined to segmental/sectional branches (Vp1-2 or Vv1); and group D, gross VI within/beyond major vascular branches (Vp3-4 or Vv2-3). The relationship between groups A–D and the clinicopathological factors is shown in Table 1.
9
Table 1. Comparison of clinicopathological data for patients classified into four groups.
Total (n=676) Group A (N=335) Group B (N=193) Group C (N=103) Group D (N=45) P value Gender 0.28 Male 530 (78.4%) 260 (77.6%) 146 (75.6%) 85 (82.5%) 39 (86.7%) Female 146 (21.6%) 75 (22.4%) 47 (24.4%) 18 (17.5%) 6 (13.3%) Ages (years) 52.3 ± 10.2 52.7 ± 10.0 52.1 ± 10.7 51.4 ± 10.5 51.7 ± 9.4 0.244 Hepatitis B surface antigen 0.171
Negative 141 (21.5%) 76 (23.2%) 43 (23.2%) 17 (17.0%) 5 (11.1%) Positive 516 (78.5%) 251 (76.8%) 142 (76.8%) 83 (83.0%) 40 (88.9%) Hepatitis C antibody 0.351 Negative 593 (94.0%) 293 (93.3%) 166 (93.3%) 91 (94.8%) 43 (100.0%) Positive 38 (6.0%) 21 (6.7%) 12 (6.7%) 5 (5.2%) 0 (0.0%) Platelet count (*1000/uL) 170 ± 81 157 ± 75 174 ± 82 187 ± 85 204 ± 96 < 0.001 Serum creatinine (mg/dL) 1.0 ± 0.9 1.0 ± 0.8 1.1 ± 1.3 0.9 ± 0.2 0.9 ± 0.2 0.49 Serum albumin (g/dL) 4.0 ± 0.5 4.0 ± 0.5 3.9 ± 0.5 4.0 ± 0.4 3.8 ± 0.5 0.368 Serum total bilirubin (mg/dL) 0.9 ± 1.2 0.9 ± 0.6 1.1 ± 1.9 0.9 ± 1.0 0.9 ± 0.5 0.587 Serum AST (U/L) 59.5 ± 66.4 50.9 ± 40.5 69.6 ± 102.9 57.3 ± 37.0 85.1 ± 65.2 0.002 Serum ALT (U/L) 56.1 ± 59.8 54.2 ± 48.3 59.9 ± 84.0 50.7 ± 38.2 66.7 ± 52.8 0.471 Prothrombin time (seconds) 12.4 ± 1.4 12.5 ± 1.4 12.5 ± 1.6 12.1 ± 1.3 12.8 ± 1.4 0.587 ICG-R15 (%) 14.9 ± 9.8 15.4 ± 9.9 13.7 ± 7.9 15.8 ± 13.2 14.3 ± 8.5 0.608 Child-Turcotte-Pugh classification 0.062 A 623 (92.6%) 317 (95.2%) 170 (88.5%) 94 (91.3%) 42 (93.3%) B 36 (5.3%) 9 (2.7%) 16 (8.3%) 8 (7.8%) 3 (6.7%)
10 C 14 (2.1%) 7 (2.1%) 6 (3.1%) 1 (1.0%) 0 (0.0%) Alpha-Fetoprotein (ng/mL) 3705.9±1089 1.1 913.7 ± 4020.0 4598.0 ± 11355.1 7336.6 ± 16371.8 12966.7 ± 18814.6 < 0.001 Preoperative TACE 0.086 No 413 (64.0%) 195 (60.7%) 126 (69.6%) 68 (68.7%) 24 (54.5%) Yes 232 (40.0%) 126 (39.3%) 55 (30.4%) 31 (31.3%) 20 (45.5%) Types of Hepatic resection < 0.001 Major 262 (38.7%) 87 (26.0%) 77 (39.9%) 57 (55.4%) 41 (91.1%) Sectionectomy 184 (27.2%) 104 (31.0%) 45 (23.3%) 33 (31.7%) 2 (4.4%) Segmentectomy or less 230 (34.1%) 144 (43.0%) 71 (36.8%) 13 (12.9%) 2 (4.4%) Size of the tumor
(cm) 5.4±3.9 3.9 ± 2.9 6.1 ± 4.2 7.2 ± 3.7 9.5 ± 4.2 < 0.001 Tumor number 0.001 Single 536 (79.3%) 287 (85.7%) 142 (73.6%) 76 (73.8%) 31 (68.9%) Multiple 140 (20.7%) 48 (14.3%) 51 (26.4%) 27 (26.2%) 14 (31.1%)
Portal vein invasion <
0.001 Negative 537 (79.4%) 335 (100.0%) 193 (100.0%) 7 (6.8%) 2 (4.4%) Positive 139 (20.6%) 0 (0.0%) 0 (0.0%) 96 (93.2%) 43 (95.6%) Hepatic vein invasion < 0.001 Negative 647 (95.7%) 335 (100.0%) 193 (100.0%) 89 (86.4%) 30 (66.7%) Positive 29 (4.3%) 0 (0.0%) 0 (0.0%) 14 (13.6%) 15 (33.3%) Microvascular invasion < 0.001 Negative 348 (51.5%) 335 (100.0%) 0 (0.0%) 9 (6.9%) 4 (4.7%)
11 Positive 328 (48.5%) 0 (0.0%) 193 (100.0%) 94 (93.1%) 41 (95.3%) Intrahepatic metastasis < 0.001 Negative 452 (66.9%) 265 (79.1%) 124 (64.2%) 48 (46.6%) 15 (33.3%) Positive 224 (33.1%) 70 (20.9%) 69 (35.8%) 55 (53.4%) 30 (66.7%) Histologic grading by Edmondson and Steiner's classification <
0.001 Negative 379 (60.7%) 227 (78.3%) 88 (46.1%) 46 (46.0%) 18 (41.9%) Positive 245 (39.3%) 63 (21.7%) 103 (53.9%) 54 (54.0%) 25 (58.1%) Microscopic resection margin < 0.001 Negative 589 (87.9%) 312 (93.7%) 170 (89.0%) 79 (77.5%) 28 (63.6%) Positive 81 (12.1%) 21 (6.3%) 21 (11.0%) 23 (22.5%) 16 (36.4%) Cirrhosis 0.346 Negative 296 (47.0%) 141 (44.8%) 93 (50.8%) 47 (50.5%) 15 (38.5%) Positive 334 (53.0%) 174 (55.2%) 90 (49.2%) 46 (49.5%) 24 (61.5%) AJCC TNM stage < 0.001 I 260 (38.4%) 265 (79.1%) 2 (1.0%) 0 (0.0%) 0 (0.0%) II 244 (36.1%) 40 (11.9%) 142 (73.6%) 65 (63.1%) 0 (0.0%) III-A 62 (9.2%) 14 (4.2%) 26 (13.5%) 24 (23.3%) 0 (0.0%) III-B 50 (7.4%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 38 (84.4%) III-C 54 (8.0%) 15 (4.5%) 21 (10.9%) 12 (11.7%) 6 (13.3%) IV-A 6 (0.9%) 1 (0.3%) 2 (1.0%) 2 (1.9%) 1 (2.2%) LCSGJ TNM stage < 0.001 I 78 (11.8%) 67 (20.6%) 11 (5.7%) 0 (0.0%) 0 (0.0%) II 329 (49.8%) 207 (63.7%) 114 (59.1%) 5 ( 5.1%) 3 ( 6.7%)
12
III 183 (27.7%) 51 (15.7%) 63 (32.6%) 54 (55.1%) 15 (33.3%) IV 71 (10.7%) 0 (0.0%) 5 (2.6%) 39 (39.8%) 27 (60.0%)
Group A, no microvascular invasion (McVI) or gross vascular invasion (VI); Group B, McVI without gross VI; Group C, VI confined to segmental/sectional branches; Group D, gross VI within/beyond major vascular branches; ICG-R15, indocyanine green retention rate at 15 minutes; TACE, transcatheter arterial chemoembolization; AJCC TNM, American Joint Committee on Cancer Tumor Node Metastasis; LCSGJ, the Liver Cancer Study Group of Japan
13
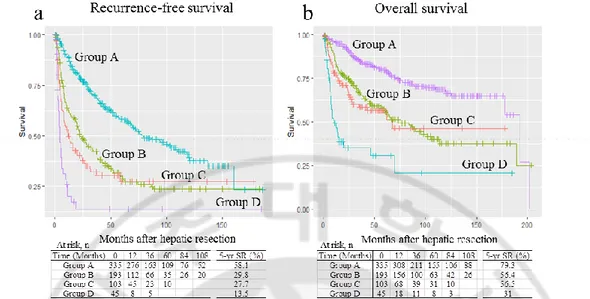
We observed marked differences in groups of patients according to preoperative platelet count, aspartate aminotransferase (AST) and AFP level, and tumor size. There were also significant differences in the percentage of major operations, multiplicity of tumors, intrahepatic metastasis, tumor histology, histological involvement of the resection margin, and tumor stage. Group D had the highest preoperative platelet counts, AST and AFP levels, and tumor sizes. In comparison, group A had the lowest values of these parameters. In addition, group D had the highest rate of major hepatic resection (91.1%), followed by group C (55.4%), whereas group A had the lowest rate of major hepatic resection (26.0%). We observed that 31.1% of patients in group D had multiple tumors, whereas 85.7% in group A had a single tumor. Moreover, group D had the highest proportions of intrahepatic metastasis and worse tumor histological grade (66.7% and 58.1%, respectively), followed by group C (53.4% and 54.0%, respectively). In addition, group D had the highest rate of positive surgical margins, followed by group C (36.4% and 22.5%, respectively), whereas group A had the lowest rate of intrahepatic metastasis (20.9%), worse tumor histological grade (21.7%), and positive surgical margins (6.3%). When comparing the survival curves according to these four groups, group D demonstrated significantly worse survival compared to the other groups: RFS and OS for groups A versus D (P < 0.001), B versus D (P < 0.001), and C versus D (P = 0.001). Moreover, groups B and C showed markedly worse outcomes than group A: RFS and OS for groups A versus B (P < 0.001) and A versus C (P < 0.001). However, no significant differences in RFS and OS were noted between groups B and C: 5-year RFS rates, 29.8% and 27.7%, respectively (P = 0.18); 5-year OS rates, 56.4% and 56.5%, respectively (P = 0.43; Figure 2). Therefore, patients were reclassified into three groups (groups A vs. B/C vs. D) for further analysis in a multivariate model.
14
Figure 4. Comparison of (a) recurrence-free and (b) overall survival of patients stratified into groups A–D. No significant changes are seen between groups B and C (recurrence-free survival, P = 0.18; overall survival, P = 0.43). SR, survival rate.
15
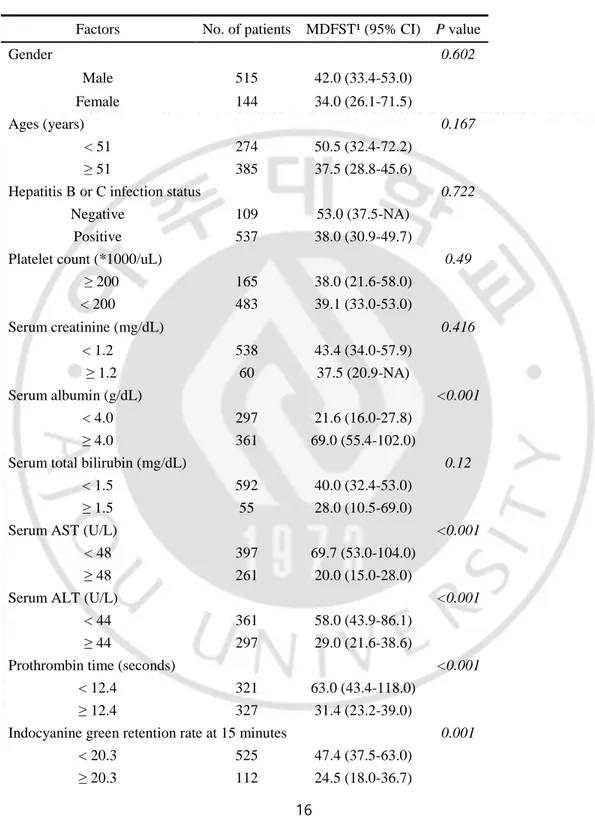
Univariate analysis according to clinicopathological factors was used to find predictors of tumor recurrence and survival. Cutoff values for the continuous variables (preoperative platelet count, AST and AFP levels, etc.) were calculated by ROC curve analysis (Table 2).
16
Table 2. Univariate analysis of factors predictive of recurrence-free and overall survival.
Factors No. of patients MDFST¹ (95% CI) P value
Gender 0.602 Male 515 42.0 (33.4-53.0) Female 144 34.0 (26.1-71.5) Ages (years) 0.167 < 51 274 50.5 (32.4-72.2) ≥ 51 385 37.5 (28.8-45.6)
Hepatitis B or C infection status 0.722
Negative 109 53.0 (37.5-NA)
Positive 537 38.0 (30.9-49.7)
Platelet count (*1000/uL) 0.49
≥ 200 165 38.0 (21.6-58.0) < 200 483 39.1 (33.0-53.0) Serum creatinine (mg/dL) 0.416 < 1.2 538 43.4 (34.0-57.9) ≥ 1.2 60 37.5 (20.9-NA) Serum albumin (g/dL) <0.001 < 4.0 297 21.6 (16.0-27.8) ≥ 4.0 361 69.0 (55.4-102.0)
Serum total bilirubin (mg/dL) 0.12
< 1.5 592 40.0 (32.4-53.0)
≥ 1.5 55 28.0 (10.5-69.0)
Serum AST (U/L) <0.001
< 48 397 69.7 (53.0-104.0)
≥ 48 261 20.0 (15.0-28.0)
Serum ALT (U/L) <0.001
< 44 361 58.0 (43.9-86.1)
≥ 44 297 29.0 (21.6-38.6)
Prothrombin time (seconds) <0.001
< 12.4 321 63.0 (43.4-118.0)
≥ 12.4 327 31.4 (23.2-39.0)
Indocyanine green retention rate at 15 minutes 0.001
< 20.3 525 47.4 (37.5-63.0)
17 Child-Turcotte-Pugh classification <0.001 A 606 45.5 (37-58.6) B or C 50 11.9 (6.0-27.7) Alpha-Fetoprotein (ng/mL) <0.001 < 12.6 223 53.0 (40.3-109.0) ≥ 12.6 426 30.2 (24.5-44.7) Preoperative TACE 0.100 No 396 51.0 (35.8-79.0) Yes 232 37.5 (26.2-48.3) Extent of resection 0.035 Major 255 31.4 (20.0-47.4) Minor 404 45.5 (36.79-61.4) Size of tumor (cm) <0.001 < 3.6 282 66.3 (51.0-99.9) ≥ 3.6 377 21.5 (16.0-28.8) Tumor number 0.317 Single 520 50.5 (38.6-66.0) Multiple 139 11.5 (8.8-21.6)
Extent of vascular invasion <0.001
Group A 326 79.0 (66.3-110.0) Group B 191 22.4 (19.3-37.2) Group C 98 12.7 (8.0-24.5) Group D 44 4.1 (3.6-7.1) Intrahepatic metastasis 0.001 Negative 443 58.0 (45.5-75.2) Positive 216 14.1 (10.4-20.5)
Histologic grading by Edmondson and Steiner's classification 0.111
I~II 370 47.4 (37.6-68.9)
III~IV 238 21.9 (16.0-37.4)
Microscopic resection margin 0.065
Negative 572 45.6 (38.0-60.4)
Positive 81 8.8 (6.0-19.3)
Cirrhosis <0.001
Negative 288 68.9 (43.4-110.0)
Positive 325 30.4 (22.2-39.9)
American Joint Committee on Cancer TNM stage <0.001
I 259 102.0 (72.2-NA)
II 242 31.6 (24.1-47.4)
18 III-B 37 4.1 (3.6-11.1) III-C 53 8.0 (4.6-14.1) IV-A 6 9.3 (7.8-NA) LCSGJ TNM stage <0.001 I 76 66.0 (51.0-NA) II 324 58.6 (45.5-94.4) III 177 20.0 (13.9-39.1) IV-A 69 5.6 (3.6-9.2)
Factors No. of patients MOST2 (95% CI) P value
Gender 0.431 Male 520 178.0 (98.0-NA) Female 145 189.0 (67.0-NA) Ages (years) 0.428 < 42 90 189.0 (64.5-NA) ≥ 42 575 178.0 (106.0-NA)
Hepatitis B or C infection status 0.964
Negative 110 193.0 (NA-NA)
Positive 541 120.0 (89.0-NA)
Platelet count (*1000/uL) 0.026
≥ 294 43 77.5 (29.4-NA) < 294 611 178.0 (109.7-NA) Serum creatinine (mg/dL) 0.55 < 1.2 542 178.0 (125.3-NA) ≥ 1.2 61 69.5 (61.2-NA) Serum albumin (g/dL) <0.001 < 4.0 300 64.2 (49.0-89.0) ≥ 4.0 364 189.0 (178.0-NA)
Serum total bilirubin (mg/dL) 0.049
< 0.8 352 193.0 (102.0-NA)
≥ 0.8 301 120.0 (83.8-NA)
Serum AST (U/L) <0.001
< 46 375 193.0 (193.0-NA)
≥46 289 59.1 (45.9-67.0)
19
< 48 407 189.0 (178.0-NA)
≥ 48 257 67.0 (56.2-110.0)
Prothrombin time (seconds) <0.001
< 12.9 392 193.0 (NA-NA)
≥ 12.9 262 88.0 (64.5-NA)
Indocyanine green retention rate at 15 minutes 0.852
< 12.9 290 178.0 (89.8-NA) ≥ 12.9 352 120.0 (84.0-NA) Child-Turcotte-Pugh classification <0.001 A 612 178 (125.3-NA) B or C 50 14 (9.7-40.2) Alpha-Fetoprotein (ng/mL) <0.001 < 16.8 261 193.0 (193.0-NA) ≥ 16.8 394 98.0 (67.2-NA) Preoperative TACE 0.017 No 403 178 (178.0-NA) Yes 231 120 (67.2-NA) Extent of resection 0.0052 Major 257 178 (69.5-NA) Minor 408 125 (101.0-NA) Size of tumor (cm) <0.001 < 5.8 447 189.0 (178.0-NA) ≥ 5.8 218 46.3 (28.0-83.8) Tumor number 0.259 Single 526 189.0 (189.0-NA) Multiple 139 34.7 (24.4-56.2)
Extent of vascular invasion <0.001
Group A 329 193.0 (178.0-NA) Group B 191 73.4 (59.1-114.0) Group C 101 67.2 (34.8-NA) Group D 44 12.0 (7.1-NA) Intrahepatic metastasis 0.049 Negative 447 189.0 (189.0-NA) Positive 218 43.0 (33.2-63.3)
Histologic grading by Edmondson and Steiner's classification 0.004
Negative 373 178.0 (125.3-NA)
Positive 241 71.4 (59.1-110.0)
20 Negative 578 189.0 (119.8-NA) Positive 81 28.7 (13.2-NA) Cirrhosis <0.001 Negative 291 193.0 (193.0-NA) Positive 328 89.0 (67.0-NA)
American Joint Committee on Cancer TNM stage <0.001
I 262 202.0 (NA-NA) II 244 98.0 (67.2-NA) III-A 63 34.9 (25.6-89.8) III-B 37 9.63 (6.8-NA) III-C 53 18.4 (12.9-83.8) IV-A 6 16.0 (4.67-NA) LCSGJ TNM stage <0.001 I 77 NA (NA-NA) II 325 202.0 (202.0-NA) III 179 64.5 (43.0-178.0) IV-A 70 13.8 (9.0-27.9)
21
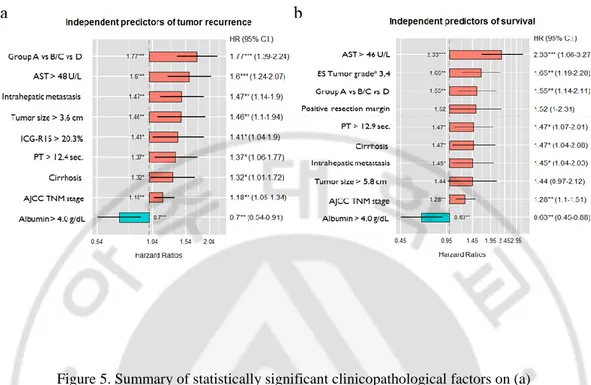
Multivariate analysis revealed predictors that were independently associated with tumor recurrence and OS. The extent of VI (groups A vs. B/C vs. D), higher AST level, existence of intrahepatic metastasis, larger tumor size, elevated ICG-R15 value, prolonged prothrombin time, liver cirrhosis, and advanced tumor stage were independent risk factors for tumor recurrence. Albumin level over 4 g/dL was a positive risk factor for prognosis (Figure 3a). Among the abovementioned risk factors, larger tumor size, elevated ICG-R15 value were not significantly related to poor OS. Worse histological grade and positive surgical margins were independent predictive factors of worse survival (Figure 3b).
22
Figure 5. Summary of statistically significant clinicopathological factors on (a) recurrence-free survival and (b) overall survival using the Cox regression proportional hazards model. AJCC TNM, American Joint Committee on Cancer Tumor Node Metastasis; AST, aspartate aminotransferase; CTP, Child–Turcotte–Pugh; ES, Edmondson–Steiner classification; HR, hazard ratio; C.I., confidence interval; ICG-R15, indocyanine green retention rate at 15 min; PT,
23 DISCUSSION
Our study demonstrated the clinical significance of McVI in a manner that has not been used in previous similar studies. When the influence of McVI was analyzed, tumor recurrence and survival rates of patients with HCC and McVI without gross VI (group B) were not different from those of patients with gross VI confined to segmental/sectional braches (group C) after hepatic resection. To the best of our knowledge, no study has directly compared the relative importance of McVI and gross VI on tumor recurrence and long-term survival of patients with HCC undergoing hepatic resection.
Previous studies have shown that McVI is an important factor affecting the prognosis of patients with HCC, especially after hepatic resection or LT. [8-11] However, it is difficult to find studies dealing with the significance of McVI as the degree of local tumor invasion or extension, despite the instinctive guess that it might be an intermediate state of VI between nonvascular invasion and gross tumor invasion of neighboring segmental vessels. We generally assumed that the risk of tumor recurrence, as well as death, would be significantly lower in patients with HCC and McVI without gross VI (group B) than in those with gross VI confined to the segmental/sectional branch (group C). Contrary to our expectation, McVI has similar prognostic power compared with gross VI confined to the segmental/sectional branch (Figure 2).
Despite its importance, official staging systems, such as the LCSGJ TNM, and BCLC staging systems, contain no mention of McVI. [6,7] The protocol developed by the College of American Pathologists considers McVI the same as gross VI of HCC on the AJCC/UICC TNM staging system, although related studies are difficult to find. [14] Then, we focused on whether tumor stage would be influenced by McVI. Our primary goal for this study has been to evaluate the importance of McVI in tumor stage for HCC. In the present study, patients with HCC and McVI without gross VI (group B) had similar outcomes of tumor recurrence and survival compared with those with gross VI confined to segmental/sectional branches (group C). When compared with patients with gross VI within/beyond major vascular branches (group D), patients in group B/C had lower rates of tumor recurrence and good survival (Figure 3). Our results tended to support the protocol of the College of American Pathologists.
24
without gross VI (group A), those with HCC with gross VI confined to segmental/sectional branches (group C) had better outcomes than those with gross VI within/beyond major vascular branches (group D). Several studies have dealt with the extent of gross VI and its clinical impact on HCC. [21,22] Survival outcomes of these previous studies are comparable to those of our study. The essential of cancer surgery is complete removal of tumor with free and safe margins. From the viewpoint of surgical principle, resection of a tumor with gross VI isolated within segmental/sectional branches could be considered as curative intention treatment through major hepatic resection without exposure of tumor thrombus margins on the portal or hepatic vein. However, resection of tumor with gross VI within/beyond major vascular branches should be considered as palliative treatment because exposure of tumor thrombus inside the vessels is not avoidable.
In the BCLC staging system, patients with HCC and VI (preoperative gross VI on image studies) are classified as having stage C disease and guided into treatments with palliative intent. [7] There is no mention of McVI because the BCLC system is designed to guide treatment according to preoperative patient information and McVI can be postoperatively confirmed through resected specimen. Therefore, there have been efforts to preoperatively detect McVI in HCC. Tools, such as performance of prothrombin induced by vitamin K absence-II and fluorodeoxyglucose-positron emission tomography, have already been suggested to preoperatively predict McVI of HCC. [23,24] Moreover, several studies have been conducted using radiological imaging, molecules or gene expression from tumor, and other preoperative tumor characteristics. [25-27] At this point, a practical question can be raised. Should patients with HCC be guided to palliative treatment if McVI can be preoperatively determined? We suggest that treatments with curative intent should be recommended for patients with HCC if they have good liver function, based on the results of our current study.
VI of HCC tumor is considered to be a reflection of aggressiveness and has a well-known negative prognostic impact after hepatic resection. [28] However, little information is available regarding this tumor progression mechanism, which remains to be elucidated. A possible postulation is that portal vein or hepatic vein tumor invasion may simply be an effect of tumor topography, which means that this aggressive phenomenon may happen only because of the close anatomical proximity to neighboring vessels. A study comparing gene expressions between primary tumors and
25
their paired portal vein tumor thrombi has demonstrated only a small difference. [29] However, studies focused on the mechanism of tumor metastasis have demonstrated the importance of phenotype changes in individual tumor cells. [30,31] Recently, genomic studies have shown that unique genes and noncoding RNAs may have an important role in this mechanism. [32,33]
Serum levels of aspartate aminotransferase (AST) are one of the important prognostic factors after hepatic resection for HCC in this study. A study demonstrated that higher AST levels are positively correlated with an influx of hepatitis B virus. [34] In this study, 78.5% of patients have chronic B-viral hepatitis. Advancing underlying liver diseases may also be related to mitochondrial injury, which leads the release of AST. [35] So, elevated AST level may be indirectly reflected the progress of hepatitis B. Multiple studies have supported that sustained viremia has a role in recurrence of hepatitis B virus-related HCC, and prevention effect of anti-viral therapy for recurrence. [36]
The present study limitations include its retrospective nature and nonrandomized design, even though the data were prospectively collected. Furthermore, there was little information on important patient perioperative status, such as antiviral drug use, postoperative progression of underlying liver disease, or exposure to other carcinogens including alcohols, which have been considered to influence tumor recurrence or de novo malignancy. External validation of meaningful findings in this study is also needed in a multicenter-organized database setting. Unfortunately, there is a lack of clarity in the definition of McVI, leading to inter- and intra-pathologist variability in the evaluation of McVI in HCC. [17] However, all tumor tissues were evaluated by one liver-specialized pathologist with over 25 years of experience in this study. There is an attempt to establish a definition of McVI in HCC, using general histopathological principles, requiring prospective validation. [37]
CONCLUSION
McVI showed similar clinical significance compared with gross VI confined to segmental/sectional branches as a risk factor for tumor recurrence and poor survival of patients with HCC. Therefore, this study recommends considering McVI when estimating the tumor stage to predict the prognosis and to plan follow-up surveillance and additional treatment for patients with
26 HCC.
27
III. Conditional survival analysis demonstrates that
recurrence risk of surgically treated hepatocellular
carcinoma evolves with time
INTRODUCTION
Hepatocellular carcinoma (HCC) is a frequently diagnosed cancer and a leading cause of cancer-related deaths worldwide. [1] Epidemiological evidence confirms that both HCC incidence and mortality increased between 2000 and 2010. [2] Surveillance of high-risk groups helps in the early detection of HCC, thus making curative treatment more likely. Hepatic resection is considered a curative treatment for early-stage HCC, especially for cases of solitary tumors and compensated liver function, [3] but the high rate of tumor recurrence is a major shortcoming and the main cause of poor outcomes. [4,5]
Accurate prediction of oncologic prognosis and the probability of tumor recurrence are important for planning follow-up surveillance and additional treatment of HHC patients. Studies evaluating patients stratified by various predictors of recurrence risk [6,7] have identified microvascular invasion (McVI) as a factor that can affect the prognosis of postoperative recurrence. Subsequent studies have focused on preoperative prediction of McVI. [8,9] Cirrhosis has also been associated with increased risk of tumor recurrence after curative treatment. [10]
Although McVI and cirrhosis have prognostic value for clinicians and patients, in the immediate postoperative period, there is little evidence that they are useful to forecast the probability of recurrence after a long tumor-free period. Patients at high risk of tumor recurrence who remain recurrence-free for long periods may not have the same risk as that at the time of surgery. Different prediction tools or methods may be needed to provide an insight into change in prognosis over time. This need has been addressed recently using the concept of conditional survival (CS) analysis. [11,12]
28
years, given a history of survival for s years. [13,14] Adjusting for the time (s years) the patient has already survived serves to increase accuracy of the estimated prognosis for the next t years. Applying this concept to recurrence-free survival, we can also get the information of recurrence-free CS. Applying CS analysis to recurrence-free survival, it can be estimated how much the risk of tumor recurrence evolves over time and whether the predictive power of well-known risk factors changes. Despite the potential benefits, no previous studies have used CS analysis to evaluate long-term HCC survival. This retrospective study analyzed recurrence-free CS in patients with hepatic resection for early-stage HCC, and assessed independent risk factors of tumor recurrence at initial presentation and changes in their predictive powers over a long recurrence-free period.
PATIENTS AND METHODS
This retrospective analysis was conducted in a series of patients with hepatic resection for HCC at the department of surgery in our institute between 1994 and 2011. Patients with radiologically confirmed solitary HCC without macrovascular invasion were defined as “early stage and curatively resectable,” [15] and were eligible for inclusion. Patients with multiple tumors, ruptured tumors, tumors with preoperatively identified macrovascular invasion, lymph node metastasis, and/or distant, including adrenal, metastasis were excluded. In all patients, the diagnosis of HCC had been pathologically confirmed. Cirrhosis was histopathologically confirmed as METAVIR stage 4. Patient data were extracted from prospectively collected medical records, which included demographics, the etiology of underlying liver disease, pathologic findings of the specimen, surgical results, and oncologic outcomes. This study was approved by the Medical Center Institutional Review Board, along with a waiver of informed consent.
Recurrence-free CS probability was estimated from Kaplan–Meier cumulative survival data. S(t) indicates the actuarial life table survival at time t, and CS indicates the probability that a patient will survive an additional y years given a survival history of x years, [11] and is calculated as CS (y|x) = S(x+y)/S(x). For example, the conditional probability of a patient who has already survived 4 years of surviving an additional year, S(1/4), is calculated by dividing the 5-year actuarial life table survival estimate, S(5), by the 4-year survival estimate, S(4); thus, S(1/4) = S(1+4)/S(4) = S(5)/S(4).
29
The decision to perform hepatic resection and the extent of the resection were based on assessment of the patient’s general condition, Child-Pugh liver function score, and indocyanine green retention rate at 15 min (ICG-R15). If the ICG-R15 value was favorable, hepatic resection was considered even when the Child-Pugh class was B. An additional prediction scoring system was used to select patients for major resection. [16] The majority of hepatic resections were intended to completely remove the portal territory of the tumor-containing region to be anatomical resection. Major resection was defined as the resection of three or more segments following the Brisbane classification; [17] minor resection involved two segments or less. If anatomical resection was not possible, we tried to obtain an appropriate margin length. Intraoperative ultrasonography was used to identify any additional lesions and to determine the optimal resection plane. Hospital mortality included inpatient deaths that were associated with hepatic resection.
After discharge, all patients were followed every 1 to 3 months during the first 2 years and every 3 months for the following 3 years. Thereafter, patients were seen twice a year. Liver function tests and serum α-fetoprotein (AFP) levels were obtained at every visit, and routine surveillance included abdominal ultrasonography or computerized tomography (CT) scans and chest radiographs every 3 months during the first 2 years and every 6 months thereafter.
Recurrence was defined as the presence of a radiologically confirmed tumor with an increase in serum AFP. If a recurrence was highly suspected without any clear imaging evidence, hepatic arteriography and lipiodol CT scans were performed. Depending on the tumor status and underlying liver function, patients with intrahepatic recurrence were treated by re-resection, local ablation, transcatheter arterial chemoembolization (TACE), or salvage liver transplantation (SLT). Treatment options for patients with extrahepatic metastatic disease included metastasectomy, TACE, radiation therapy, and targeted therapy with sorafenib.
Statistical analysis
All continuous variables were expressed as mean ± standard deviation or median (minimum-maximum range). Data were analyzed using SPSS 18.0 for Windows (SPSS, Chicago, Illinois, USA). Cumulative survival was estimated by the Kaplan–Meier method, and the rates were compared using the log rank test. Multivariate analysis and the Cox regression proportional hazards model were used to identify factors that independently predicted recurrence-free survival. Variables
30
significantly related to survival in the Kaplan–Meier analysis were used to stratify the study population by risk of recurrence after hepatic resection, and the Kaplan–Meier survival data were used to estimate 5-year CS. P-values <0.05 were considered statistically significant.
RESULTS
A total of 649 patients experienced primary hepatic resection for HCC during the study period. Twenty-eight were diagnosed with combined HCC and intrahepatic-cholangiocarcinoma, and 185 with multiple tumors, ruptured tumors, macrovascular invasion and/or distant metastasis including adrenal metastasis discovered during preoperative evaluation were excluded (Figure 4).
31
Figure 6. Flow diagram showing the selection of patients who were eligible for this study. HCC; hepatocellular carcinoma; CCC, cholangiocarcionoma.
32
The characteristics of the remaining 436 patients with solitary HCC and no macrovascular invasion on preoperative imaging are summarized in Table 1. There was a preponderance of male patients (340, 78%); the median age was 52 years (range 20–76 years), 324 patients (74.3%) tested positive for hepatitis B surface antigen, and 26 (6.0%) tested positive for hepatitis C virus. Four hundred and fourteen patients (95.2%) were Child–Pugh class A and 19 (4.4%) were class B. Major hepatic resection of three or more Couinaud’s segments was performed in 129 patients (29.6%).
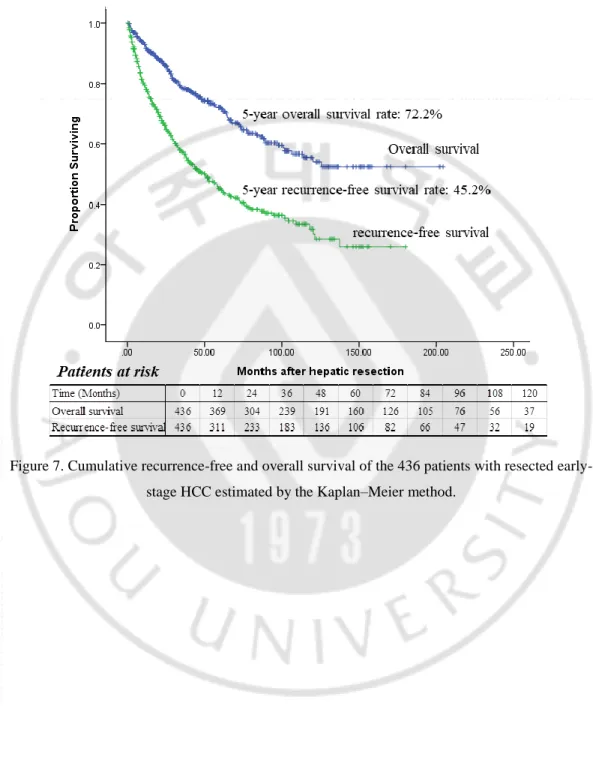
The median follow-up was 41.3 months (range 1–186). At the time of data collection, 127 patients (19.6%) had died. Four (0.92%) patients died of preoperative complications within the same hospitalization period; one of the four cases given an SLT, but did not recover. Eight died of non-liver-related causes, including another type of cancer, pneumonia, or heart attack. Twelve died of advanced liver cirrhosis, four of whom had tumor recurrence. The remaining 103 patients (83.7%) died following tumor recurrence. At the time of analysis, 342 patients were alive, and of those, 192 had not experienced a recurrence; 117 had recurrences. Median overall survival was 41 months (range: 1–204); the 5-year survival rate was 72.2% (Figure 5).
33
Figure 7. Cumulative recurrence-free and overall survival of the 436 patients with resected early-stage HCC estimated by the Kaplan–Meier method.
34
At the time of data collection, a total of 224 patients, including both those who were alive and those who had died, had experienced tumor recurrences. The median time to recurrence was 26 months (range 1–180). The rate of recurrence-free survival was 66.0% at 2 years and 45.2% at 5 years (Figure 5). Of the 224 tumor recurrences, 92 (41.1%) occurred within the first year and 137 (61.2%) occurred within 24 months of the initial hepatic resection. Fifty (22.3%) tumor recurrences occurred between the second and fifth postoperative years; the remaining 37 (16.5%) occurred more than 5 years after hepatic resection. The patterns of tumor recurrence differed depending on when they occurred. Of the recurrences that occurred within 24 months after the initial hepatic resection, 55% were solitary or oligonodular intrahepatic lesions and 45% were diffuse intrahepatic or distant metastatic lesions. The types of tumor recurrence that occurred after 24 months were either solitary or oligonodular intrahepatic lesions (80.6%) or diffuse intrahepatic or distant metastatic lesions (19.4%, P = 0.002).
Ten-year recurrence-free survival data were used to calculate CS probability. Figure 6 shows the 5-year recurrence-free CS probability and the changes in CS with each additional year without recurrence. The 5-year recurrence-free CS probability increased by 14.5%, from 58.2% to 66.6% between 2 and 5 years after hepatic resection. The univariate predictors of tumor recurrence are listed in Table 3. Multivariate analysis revealed five predictors that were independently associated with tumor recurrence. These were ICG-R15 >20%, tumor size >5 cm, presence of microvascular invasion, positive microscopic resection margin, and background liver cirrhosis (Table 3). Based on the multivariate analysis results, patients were stratified by low (having no predictor), intermediate (having one predictor), and high (having two or more predictors) recurrence risk. Figure 7a shows the recurrence-free survival in the three risk groups, which had significantly different prognoses (P <0.001). Figure 7b shows the estimated 5-year recurrence-free CS probability and the changes in CS in each risk group with each additional year of recurrence-free survival. Interestingly, a favorable 70.5% increase in 5-year recurrence-free probability occurred in the high-risk group between the second (46.8%) and fifth (79.8%) years after hepatic resection. In the low or intermediate risk groups, there were no significant changes in CS probability over time, but the 5-year recurrence-free CS probability in the low risk group decreased from 82.3% in 5-year 2 to 55.8% in year 5. Given the recurrence-free survival in the 4 years after hepatic resection, the resulting 5-year CS recurrence-free probability was nearly identical or even reversed between the risk groups.
35
Figure 8. Five-year recurrence-free conditional survival (CS) at each additional year after hepatic resection.
36
Table 3. Univariate and multivariate analysis to identify prognostic factors associated with tumor recurrence. Factors No. of patients MDFST¹ (95% CI) P value Odds ratio (95% CI) P value Clinical factors Gender 0.694 Male 340 52.5±5.82 (41.09-63.91) Female 96 37.57±7.56 (22.75-52.40) Age 0.915 ≤65 387 51.0±5.81 (39.60-62.40) >65 49 37.5±3.00 (31.62-43.38)
Hepatitis B or C infection status 0.08
No 89 72.87±22.26 (29.25-116.49) Yes 347 43.93±6.20 (31.76-56.10) Child-Pugh classification 0.268 A 415 52.0±6.21 (39.82-64.18) B or C 19 37.37±15.08 (7.81-66.93) Alpha-Fetoprotein (ng/L) 0.022 ≤9 139 56.53±21.55 (14.29-98.77) >9 291 43.93±7.72 (28.80-59.06) ALT (IU/dL) 0.637 ≤40 225 63.0±11.70 (40.07-85.94) >40 210 43.30±5.44 (32.64-53.96) ICGR15 (%) 0.001 1.829 (1.292-2.588) 0.001 ≤20 350 58.13±8.23 (41.99-74.27) >20 72 29.0±5.45 (18.31-39.69) Preoperative TACE 0.857 No 279 52.5±8.13 (36.57-68.44) Yes 137 45.5±7.82 (30.18-60.83) Pathologic factors Size of tumor (cm) 0.002 1.867 (1.334-2.613) <0.001 ≤5 316 58.0±6.80 (44.68-71.32)
37 >5 120 22.2±3.46 (15.43-28.97) Microvascular invasion <0.001 1.891 (1.397-2.560) <0.001 Abscent 262 72.87±14.56 (44.34-101.40) Present 174 24.10±3.20 (17.83-30.37) Macrovascular invasion <0.001 No 388 53.0±6.39 (40.47-65.53) Yes 48 16.0±5.02 (6.17-25.83) Intrahepatic metastasis 0.005 No 375 53.0±6.42 (40.41-65.59) Yes 61 20.50±7.42 (5.99-35.00)
Histologic grading by Edmondson and Steiner's classification 0.016
I-II 174 66.30±12.22 (42.37-90.29)
III or IV 223 37.57±5.88 (26.05-49.09)
Extent of resection 0.639
Major 129 52.00±16.50 (19.67-84.33)
Minor 307 49.67±6.47 (36.99-62.34)
Microscopic resection margin <0.001 1.915
(1.178-3.113) 0.009 Negative 397 53.00±6.30 (40.65-65.36) Positive 34 14.00±2.94 (8.25-19.75) Cirrhosis2 <0.001 1.74 (1.285-2.356) <0.001 No 199 86.00±19.06 (48.64-123.36) Yes 212 37.37±5.30 (26.98-47.76) Japanese TNM stage <0.001 I 68 60.40±10.19 (40.42-80.38) II 285 56.53±9.26 (38.37-74.69) III 67 27.73±10.38 (7.39-48.07) IVA 16 6.00±1.26 (3.54-8.46)
38
Figure 9. Recurrence-free survival curves (a) and five-year recurrence-free CS probability (b) in three risk groups. The low-risk group had no independent predictors, the intermediate-risk group had one predictor, and the high-risk group had two or more predictor
39
The adjusted 5-year recurrence-free CS probability was calculated for patients with or without an independent prognostic factor at hepatic resection. For patients with tumors <5 cm, the 5-year recurrence-free CS probabilities were 53.3% at year 1 and 59.5% at year 5. For patients with tumors >5 cm, the corresponding CS probabilities were 53.7% and 94.0%. The increase in 5-year recurrence-free CS probability was thus much greater in patients with large tumors (a 75% increase from 53.7% to 94.0%) than in those with small tumors (an 11.6% increase from 53.3% to 59.5%, Figure 8a). The differences in recurrence probability associated with preoperative ICG-R15 above or below 20% and by the absence or presence of microvascular invasion were similar to those seen for tumor size. In patients stratified by ICG-R15, the 1 year probabilities of recurrence were 58.8% and 23.9%, but by 5 years, the difference (66.7% vs. 66.5%) had nearly disappeared. The increase in 5-year recurrence-free CS probability was thus much larger in patients with preoperative ICG-R15 values >20% (a 178% increase from 23.9 to 66.5%) than in those with values <20% (a 13% increase from 58.8 to 66.7%, Figure 8b) The increase in 5-year recurrence-free CS probability in patients with microvascular invasion was 82.2% ( from 41.7% to 75.1%) and 16.5% (from 58.9% to 63.6%) in those without microvascular invasion (Figure 8c). However, the trend in change in 5-year recurrence-free CS probability was different in patients with or without background liver cirrhosis. The 5-year recurrence-free CS probability was higher in patients without baseline cirrhosis than in those with it. The increases in 5-year recurrence-free CS probability from the first to fifth years in the two groups were not significantly different. The increase in the non-cirrhosis group was 9.7% (62.6% to 68.7%) and 33% in the cirrhosis group (44.7% to 59.6%, Figure 8d).