저작자표시-비영리-동일조건변경허락 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 동일조건변경허락. 귀하가 이 저작물을 개작, 변형 또는 가공했을 경우 에는, 이 저작물과 동일한 이용허락조건하에서만 배포할 수 있습니다.
Effect of Combination of Aspirin and TRAIL in
HeLa Cervical Cancer Cells
by
Se-Ran IM
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Effect of Combination of Aspirin and TRAIL in
HeLa Cervical Cancer Cells
by
Se-Ran IM
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for the
Degree of Master of Biomedical Sciences
Supervised by
Young-Ju Jang, Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Se-Ran IM is approved.
SUPERVISORY COMMITTEE
Young-Ju Jang
Seon-Yong Jeong
You-Sun Kim
The Graduate School, Ajou University
December, 20th, 2011
i
-ABSTRACT-
Effect of Combination of Aspirin and TRAIL in HeLa Cervical
Cancer Cells
Purpose: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of
the tumor necrosis factor (TNF) family of cytokines and induces apoptosis in most tumor cells. Although TRAIL is considered a promising drug for cancer therapies because of its tumor selectivity, many tumors are resistant to TRAIL. Thus TRAIL-resistant cancer cells must be sensitized first to become responsive to TRAIL. In this study, I studied whether pretreatment by aspirin augmented TRAIL-induced apoptotic death in HeLa cells, which derived from cervical cancer and investigated the underlying mechanism.
Methods: Cell viability and proliferation were assessed by MTT assay and cytotoxicity was
analyzed by LDH assay. Annexin-V /PI staining and sub G1 analysis were used for evaluation of apoptotic cells. We measured mitochondrial membrane potential in HeLa cells undergoing apoptosis by using JC-1. Protein level changes were documented by western blot analysis.
Results: Aspirin inhibited proliferation of HeLa cells in the time- and dose-dependent
manners. The combined treatment of aspirin with TRAIL strongly enhanced TRAIL-induced apoptotic cell death in HeLa cells. It activated 8, -9 and -3 and caused the caspase-dependent loss of MMP and the release of cytochrome c from mitochondria to cytosol. The apoptotic characteristics enhanced by the combined treatment were inhibited by a pan-caspase inhibitor z-VAD-fmk. Interestingly, TRAIL caused the activation of ERK, whereas the ERK activation was blocked by aspirin. As a result, the activation of ERK decreased and additionally, Mcl-1 also decreased in combination treatment.
Conclusion: TRAIL in combination with aspirin significantly increase apoptosis in HeLa
cells through caspase-and mitochondrial-dependent pathway. Mechanism of such a promoted apoptotic effect might be associated with that TRAIL-induced ERK activation which triggers survival signal to HeLa cells is blocked by pretreatment of aspirin. Inhibition of ERK activation enhances TRAIL-induced caspase activation, which rapidly cleavages Mcl-1. It could also trigger translocation of cleaved Bid to mitochondria to activate mitochondrial
ii
pathway, which also amplifies caspase activation. Eventually, pre-treated aspirin could block the survival signal and enhance caspase-and mitochondrial-dependent apoptotic cell death in HeLa cells treated with TRAIL.
iii
TABLE OF CONTENTS
ABSTRACT ... ⅰ TABLE OF CONTENTS ... ⅲ LIST OF FIGURES ... ⅴ Ⅰ.
INTRODUCTION ... 1Ⅱ. MATERIALS AND METHODS ... 3
A. Reagents ... 3
B. Antibodies ... 3
C. Cell culture ... 3
D. Cell viability assay ... 3
E. Cytotoxicity assay ... 4
F. Sub G1 analyses ... 4
G. Annexin-V/ PI staining ... 4
H. Immunoblotting ... 5
I. Measurement of mitochondrial membrane potential ... 5
J. Analysis of cytochrome c release ... 6
Ⅲ. RESULTS ... 7
1. Aspirin inhibits the proliferation of HeLa cells and augments TRAIL-mediated cell death ... 7
2. Aspirin triggers TRAIL-induced apoptotic cell death via enhanced caspase activity 10 3. Aspirin results in TRAIL-induced caspase-dependent mitochondrial membrane potential change and cytochrome c release ... 16
4. Aspirin makes TRAIL-resistant HeLa cells TRAIL-sensitive through inhibition of ERK1/2 activation ... 19
Ⅳ. DISCUSSION ... 26
iv
Ⅴ. REFERENCES ... 29 Ⅵ. 국문요약 ... 36
v
LIST OF FIGURES
Fig. 1. Effect of the combined treatment of aspirin with TRAIL on the proliferation and
cytotoxicity of HeLa cells ... 8
Fig. 2. Morphologies of HeLa cells treated with aspirin, TRAIL or combination of them in the presence or absence of z-VAD ... 9
Fig. 3. Aspirin enhanced TRAIL-induced apoptosis ... 12
Fig. 4. Pretreatment of aspirin enhanced TRAIL-induced apoptosis ... 13
Fig. 5. The combination treatment of aspirin (ASA) and TRAIL promoted caspase activity ... 14
Fig. 6. Apoptotic cell death induced by the combination of aspirin and TRAIL was inhibited by z-VAD ... 15
Fig. 7. The combination of aspirin and TRAIL induced MMP change ... 17
Fig. 8. A pan-caspase inhibitor, zVAD-fmk, prevented cytochrome c release from mitochondria of the combination treated cells ... 18
Fig. 9. Effect of various time of aspirin treatment and combined treatment of aspirin with TRAIL ... 20
Fig.10. Effect of the combined treatment of PD98059 with TRAIL ... 22
Fig.11. Effect of caspase about clevage of Mcl-1 ... 23
Suppl. 1. Cytotoxicity was analyzed by LDH assay ... 24
Suppl. 2. Morphologies of HeLa cells treated with aspirin, TRAIL or combination of them in the presence or absence of z-IETD ... 25
- 1 -
Ⅰ. INTRODUCTION
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an apoptosis-inducing member of the TNF gene superfamily and has attracted attention not only for its strong antitumor activity but also for its minimal cytotoxity to most normal cells and tissues (Pitti et al., 1996). The potential of TRAIL as a chemotherapeutic agent is limited, however, because of the emergence of TRAIL resistance (Bouralexis et al., 2003, Tillman et al., 2003, Kang et al., 2009). Thus, TRAIL-resistant cancer cells must be sensitized first to become responsive to TRAIL. Several researchers have reported that TRAIL resistance can be overcome by various sensitizing agents such as chemotherapeutic drugs (Lee et al., 2001, Fulda et al., 2004, Jane et al., 2011), cytokines (Park et al., 2002, Mezosi et al., 2004, Büneker et al., 2011), and matrix metalloprotease inhibitors (Nyormoi et al., 2003).
There are two distinct routes of TRAIL-induced apoptotic signal pathway— the 'extrinsic' and 'intrinsic' pathways, which proceed through death receptors and through mitochondrial events, respectively. The intrinsic pathway of apoptosis regulates the activity of the Bcl-2 family proteins that control the integrity of the mitochondrial membrane. The release of proapoptotic factors from the mitochondria, such as cytochrome c, into the cytoplasm promotes the activation of initiator caspase-9, which in turn activates effector caspases such as caspase-3 or -7 (Green and Reed, 1998, Gross et al.,1999, Wang et al.,2001). The extrinsic pathway relies on the activation of death receptors from the TNF receptor family that promote the recruitment and activation of initiator caspase-8 via adaptor proteins such as Fas-associated death domain (FADD) or TNFRSF1A-associated death domain (TRADD) (Strasser et al., 2000, Ashkenazi et al., 2002). Strong caspase-8 activity may directly activate effector caspases; it may also require signal amplification through induction of the intrinsic pathway via cleavage of the Bcl-2 family protein Bid (LeBlanc et al., 2002, LeBlanc and Ashkenazi, 2003, Broaddus et al., 2005, Li et al., 1998, Luo et al., 1998). However, TRAIL signaling does not only lead to the activation of effector caspases and subsequent initiation of apoptosis, but can also induce non-apoptotic pathways, which includes the activation of NF-kB, PKB/Akt and MAPKs (Degli et al.,1997, MacFarlane, 2003). TRAIL exposure
- 2 -
results in the activation of ERK1/2 and the elevation of anti-apoptotic Bcl-2 family protein levels. ERK1/2 activation is quite active in the mechanisms underlying protection against TRAIL-induced signaling (Sale et al.,1995, Marshall et al.,1995, Lee et al., 2006).
Aspirin (acetylsalicylic acid, ASA) and other nonsteroidal anti-inflammatory drugs (NSAIDs) are used clinically for their anti-inflammatory, anti-pyretic, and analgesic properties. Pharmacologically, NSAIDs is known to act directly suppressing cyclooxygenase enzymes (COX-1 and COX-2) in various cell types and tissues. NSAIDs inhibit the proliferation rate, alter the cell cycle distribution and induce apoptosis in colon cancer cell lines (Chan, 2003, Bertagnolli, 2003, M.Gloria et al., 2007). Also, aspirin is known to significantly block ERK activity (Shuanglin et al., 2010).
In this study, I examined whether pretreatment by aspirin augmented TRAIL-induced apoptotic death in HeLa cells, which derived from human cervical cancer and investigated the underlying mechanism. It is postulated that modulation of ERK activity by aspirin contributed to sensitize the TRAIL-resistant HeLa cells. It is demonstrated that aspirin augments TRAIL-induced apoptosis by inhibition of ERK1/2 activation and subsequently by increasing caspase activation.
- 3 -
Ⅱ. MATERIALS AND METHODS
A. Reagents
Aspirin was purchased from Sigma-Aldrich and dissolved in DMSO and the pH was adjusted to 7.0 using 10N NaOH. Recombinant human TRAIL/Apo2 ligand (ten non-tagged 19KDa protein, amino acid 114-281) was obtained from KOMA Biotech (Seoul, South Korea). ERK inhibitor PD98059 and zVAD-fmk were purchased from Cell signaling.
B. Antibodies
Antibodies were purchased from the companies as follows- Cell Signaling: anti-cCaspase-3, anti-Caspase-8, anti-Caspase-9, anti-PARP, ant-Bid, ant-Bak, anti-Mcl-1, Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), and ERK. Clontech: cox4 and anti-cytochrome c. Santa Cruz: anti-p53. Epitomics: anti-Bax and anti-Bcl-2. Abcam: anti-DR5, and goat polyclonal secondary antibody to rabbit IgG-H&L (HRP), Upstate: anti-TRAILR1 and Abfrontier: anti-α-tubulin.
C. Cell culture
Human adenocarcinoma HeLa cells were obtained from the American Type Culture Collection. HeLa cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco-BRL, Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Gibco-BRL). The dishes containing cells were kept in a 37°C humidified incubator with a mixture of 95% air and 5% CO2.
D. Cell viability assay
Inhibition of cell proliferation was evaluated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide (MTT, Sigma, St. Louis, MO, USA). Briefly, cells were seeded (5 х103) in 96-well flat-bottomed plates in a final volume of 100ul. After attachment for 20 h, cells were pretreated with aspirin (5mM) for 24hr and then TRAIL (100ng/ml) treatment for 8hr or indicated period of time. After drug exposure, 10ul of MTT (5 mg/ml
- 4 -
in D.W) solution were added and incubated in 37°C incubator for an additional 3 hr. The reaction was stopped by removal of MTT, and formazan crystals were solubilized in 100 ul dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO, USA) in each well. Absorbance at 570 nm was recorded using a Model 680 Microplate Reader (Bio-Rad, Bath, UK).
E. Cytotoxicity
LDH is a stable cytosolic enzyme that is released upon membrane damage. LDH activity was measured using a commercial cytotoxicity assay kit (Promega), in which released LDH in culture supernatants is measured with a coupled enzymatic assay, resulting in conversion of a tetrazolium salt into red formazan product. The cells were treated with 5mM aspirin and/or 100ng/ml TRAIL for indicated period of time. The sample solution (supernatant) was removed and LDH released from cells was measured in culture medium. Absorbance at 490nm was recorded using a Model 680 Microplate Reader (Bio-Rad, Bath, UK).
F. Sub G1 analyses
Propidium iodide (PI) staining and flow cytometry were used to determine the distribution of cell cycle and degree of apoptosis. Cells (2 x 105) were washed with 1ml phosphate-buffered saline (PBS), then treated by 1ml 0.05% trypsin-EDTA at 37°C for 3 min. Cells were washed with 1ml PBS and resuspended in 50 ul PBS. Resuspended cells were added dropwise into 1ml of cold 70% ethanol with gentle vortexing, then kept at -20°C for overnight. The fixed cells were centrifuged at 400 g at 4 °C for 10 min, and pellets were washed with 1ml PBS and resuspended in 50 μl PI (10 μg/ ml) containing RNase (300μg/ml). Then, the cells were stored at 37°C for 60 min and analyzed by a flow cytometer using FACS Aria III and Diva program (Becton Dickinson, CA, USA).
G. Annexin-V/ PI staining
Apoptosis-mediated cell death of tumor cells was examined using a double staining method with FITC-labeled Annexin V/PI Apoptosis Detection kit (BD Bioscience) according to the manufacturer's instructions. To summarize briefly, cells (2 x 105) were washed with PBS and obtained by trypsinization. Cells were sedimented by centrifugation
- 5 -
(1500rpm /5min), washed with PBS, resuspended in 200 μl 1xbinding buffer and incubated with 5 μl Annexin V-FITC and 5 μl PI for 15min. Cells were analyzed immediately using a FACS flow cytometer (FACS Calibur BD Biosciences, Heidelberg, Germany). Cells in early stages of apoptosis are presented by Annexin V single positivity; whereas, cells that are Annexin V/PI double positive present the late stage of apoptosis.
H. Immunoblotting
Cells were washed in PBS and lysed with 2X Laemmli buffer (126 mM Tris-HCl [pH6.8], 4% SDS, 20% glycerol, 0.02% Bromphenolblue and 2% b-mercaptoethanol) and boiled for 5 min. The samples were separated by 8-15% SDS-polyacrylamide gels, and transfered to Immobilon membranes (Millipore). The membranes were blocked with 5% nonfat dry milk in TBS/Tween20 (0.05%, v/v) for 1h, incubated for 4°C overnight with primary antibodies. The membranes were then washed three times with TBST buffer (10 mM Tris-HCl [pH7.4], 150 mM NaCl, 0.05% Tween 20) and incubated further for 1 h with horseradish peroxidase–conjugated anti-rabbit or mouse antibody. Visualization of protein bands was accomplished using enhanced chemiluminescence (Amersham Life Science).
I. Measurement of Mitochondrial Membrane Potential (MMP)
The integrity of the mitochondrial membrane (∆Ψm) was evaluated with the cationic dye,
5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimi-dazolylcarbocyanineiodide,JC-1(Invitrogen). Cells (2 × 105) were trypsinized and washed with PBS twice. The cell pellets were incubated
at 37°C for 30 minutes in 1 mL complete medium containing 1 ul JC-1 stock solution (5mg/ml). The cells were washed with PBS and resuspended with 300ul PBS. The mean green fluorescence (FL-1 channel) and mean orange-red fluorescence (FL-2 channel) were recorded and quantified by flow cytometry (FACS Aria III and DIVA software; BD Biosciences). The Ψm was expressed as the mean red to mean green ratio. Unstained control cells were included to evaluate baseline fluorescence.
- 6 -
J. Analysis of cytochrome c release
To assess the mitochondrial cytochrome c release in HeLa cells following combination treatment, cytosolic protein extracts were obtained according to the cell fractionation kit user manual (Clontech). Briefly, 1х106 cells in 60∅ dish were washed twice with cold PBS and centrifuged at 600 х g for 5 min at 4°C. Then, the cells were suspended in 0.1 ml of ice-cold fractionation buffer mix. The cells were allowed to swell on ice for 10 min and homogenized with a syringe needle. The homogenates were centrifuged at 700 х g for 10 min at 4°C, and the supernatants were centrifuged at 10,000 х g for 25 min at 4°C. The supernatants were harvested as cytosolic extracts free of mitochondria, and analyzed for cytochrome c release from mitochondria.
- 7 -
Ⅲ. RESULTS
1. Aspirin inhibits the proliferation of HeLa cells and augments TRAIL-induced cell death
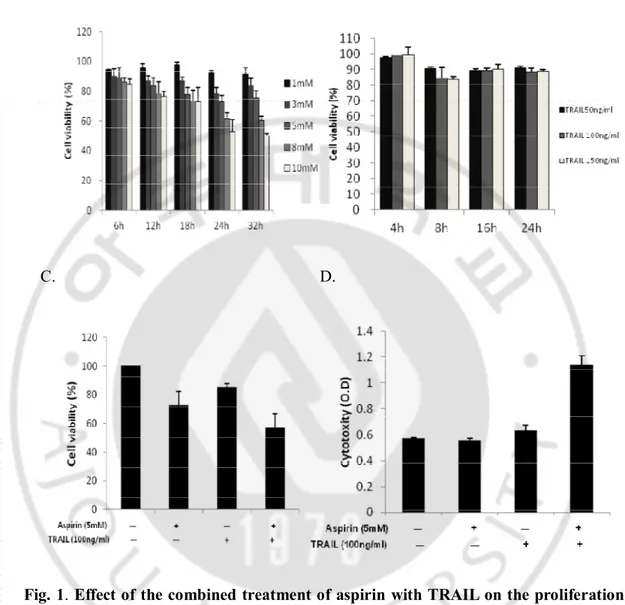
Firstly, in order to investigate inhibitory effect of aspirin on the proliferation of HeLa cells, the cells were treated with various concentrations of aspirin (0, 1, 3, 5, 8, and 10 mM) for different time (0, 6, 12, 18, and 24hr) (Fig. 1A). Cellular proliferation was assessed by MTT assay. It was shown that proliferation of HeLa cells treated with aspirin was obviously inhibited in the time- and dose-dependent manners. We also investigated the cytotoxic effect of TRAIL at the concentrations of 50 – 150 ng/ml on the cells. HeLa cells were relatively resistant to TRAIL mediated cytotoxicity at these concentrations (Fig. 1B). To investigate the effect of combination treatment of aspirin (5mM) and TRAIL (100ng/ml), on the cytotoxicity both MTT and LDH assays were used (Fig. 1C and D). LDH assay assesses LDH release into the culture medium as a marker of dead cells. Aspirin (5mM) alone did not induce cell death, but inhibited cell proliferation. TRAIL (100ng/ml) also did not induce cell death remarkably in LDH assay, which was shown in MTT assay as well. But in case of the combination of aspirin and TRAIL, the rate of cell death considerably increased (Fig. 1D). Enhanced cell death by the combination was also shown by microscopic observation in a time-dependent manner (Fig. 2).
- 8 - A. B.
C. D.
Fig. 1. Effect of the combined treatment of aspirin with TRAIL on the proliferation and cytotoxicity of HeLa cells. HeLa cells were incubated with various concentrations of
aspirin (A) or TRAIL (B) in different times as described. HeLa cells were pretreated with aspirin (5mM) for 24h and then treated with TRAIL (100ng/ml) for 8h (C and D). Cell viability was analyzed by MTT assay (C). Cytotoxcity was anayzed by LDH assay (D). Cells were seeded at 5 х 103 cells per well of 96-well microtiter plates and stabilized for 20hr, before treatment with aspirin alone, TRAIL alone , or combination of aspirin and TRAIL.
- 9 -
Fig.2. Morphologies of HeLa cells treated with aspirin, TRAIL or combination of them in the presence or absence of z-VAD. HeLa cells (2 х 105) were plated onto 6-well tissue culture plates and stabilized for 20hr. Cells were pretreated with aspirin(ASA) (5mM) for 24hr and treated continuously with TRAIL (100ng/ml) for 4, 6, or 8hr without changing media. For the treatment of z-VAD, the cells were pretreated with z-VAD (50uM) for 1hr and followed by treatment with aspirin, TRAIL or the combination.
- 10 -
2. Aspirin triggers TRAIL-induced apoptotic cell death via enhanced caspase activity
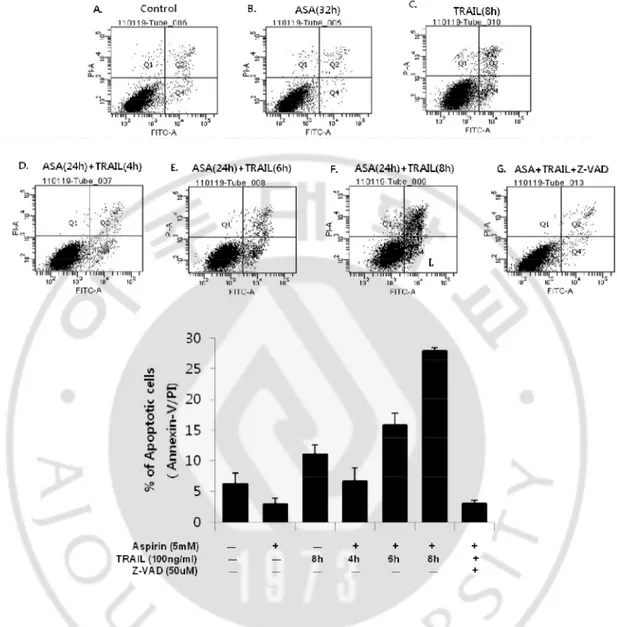
To see the type of cell death, we performed annexin-V-FITC / PI double staining for the measurement of phosphatidylserine (PS) exposure. PI staining was also done for the measurement of sub-G1 population (Fig. 3 and Fig. 4). The annexin-V-FITC/PI staining for flow cytometry is the most commonly used method for detecting apoptosis. Annexin-V is a calcium-dependent phospholipid binding protein that has a high affinity for the PS, a plasma membrane phospholipid. One of the earliest features of apoptosis is the translocation of PS from the inner to the outer leaflet of the plasma membrane, thereby exposing PS to the external environment. Annexin-V binds to PS exposed on the cell surface and identifies cells at an earlier stage of apoptosis than assays based on DNA fragmentation. The apoptotic cells with degraded DNA appear as cells with hypodiploid DNA content and are represented in so-called "sub-G1" peaks on DNA histograms.
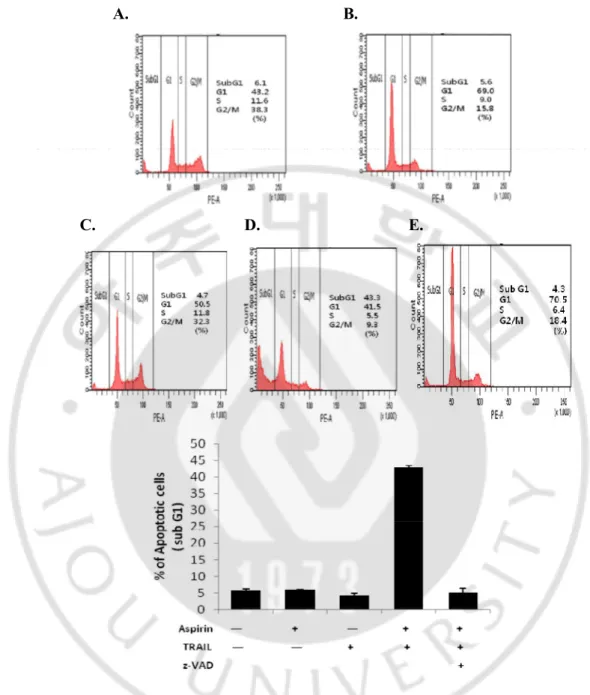
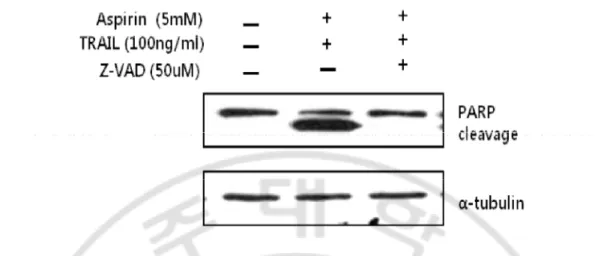
Early apoptotic cells detected by annexin-V-FITC were only 0.5% and 5% in aspirin and TRAIL alone treatment, respectively. However, they presented 3.2%, 7.3%, or 11.0% when the cells were pre-treated with aspirin for 24hr and continuously treated with TRAIL for 4hr, 6hr, or 8hr (Fig.3D, E, F and G), indicating that aspirin promoted TRAIL–induced apoptotic cell death in a time-dependent manner. Percentage of sub-G1 population was low in the untreated control cells (6.1%) (Fig.4A). Percentage of sub-G1 population in TRAIL-treated cells (4.7%) (Fig. 4C) was significantly increased in co-treated cells with TRAIL and aspirin (43.3%) (Fig. 4D and F). Next, we examined whether apoptotic cell death induced by the combined treatment depends on caspase activities. First we performed western blotting analysis. The combination markedly induced the activation of caspase-8, -9, and -3 (Fig. 5). To further confirm that caspase activation is required for the increased apoptosis in combination treated HeLa cells, a pan-caspase inhibitor zVAD-fmk (50uM) was added to the cells 1 hour before treatment with aspirin for 24 hours and TRAIL for 8 hours. They were analyzed by flow cytometry for annexin-V-FITC staining, and the microscope observation was also performed (Fig. 2, Fig. 3C, and Fig. 4E). The characters associated with apoptosis, such as cell morphology, exposure of PS, subG1 population and PARP cleavage, were all completely disappeared by a pan-caspase inhibitor, zVAD-fmk. These results strongly
- 11 -
suggest that aspirin promotes TRAIL-induced apoptotic cell death through enhancement of caspase activation.
- 12 -
Fig. 3. Aspirin(ASA) enhanced TRAIL-induced apoptosis. A.HeLa cells were untreated.
B. Aspirin was treated for 32hr. C. TRAIL was treated for 8hr. D~F. Aspirin was treated for 24hr and TRAIL was continuously treated for 4hr (D), 6hr (E) and 8hr (F). G. z-VAD was pretreated for 1hr and followed by treatment of aspirin and TRAIL for 24hr and 8hr, respectively. Concentrations of aspirin, TRAIL and z-VAD-fmk were 5mM, 100ng/ml and 50uM, respectively. HeLa cells were seeded at 2 х 105 cells per well of 6-well culture plate and stabilized for 20hr before any treatment.
- 13 -
A. B.
C. D. E.
Fig. 4. Pretreatment of aspirin enhanced TRAIL-induced apoptosis. A.Untreated cells. B.
Aspirin was treated for 32hr. C. TRAIL was treated for 8hr. D.E. Aspirin was treated for 24hr and followed by treatment of TRAIL for 8hr with (E) or without (D) pretreatment of 50uM zVAD-fmk for 1hr, respectively. HeLa cells were seeded at 2 х 105 cells per 6-well plate and stabilized for 20hr. Concentrations of aspirin and TRAIL were 5mM and 100ng/ml, respectively.
- 14 -
Fig.5. The combination treatment of aspirin and TRAIL promoted caspase activity.
HeLa cells were seeded at 2 х 105 cells per 6-well plate and stabilized for 20hr. Aspirin was treated for 24hr and followed by TRAIL treatment for 8hr. Concentrations of aspirin and TRAIL were 5mM and 100ng/ml, respectively. Cells were harvested and equal amount of protein was processed for western blot analysis to detect target proteins.
- 15 -
Fig. 6. Apoptotic cell death induced by the combination of aspirin and TRAIL was inhibited by z-VAD. HeLa cells were pretreated with 50uM of z-VAD for 1hr and aspirin
and TRAIL were treated as previously described. Concentrations of aspirin and TRAIL were 5mM and 100ng/ml, respectively. Cells were harvested and equal amount of protein was processed for western blot analysis to detect target proteins.
- 16 -
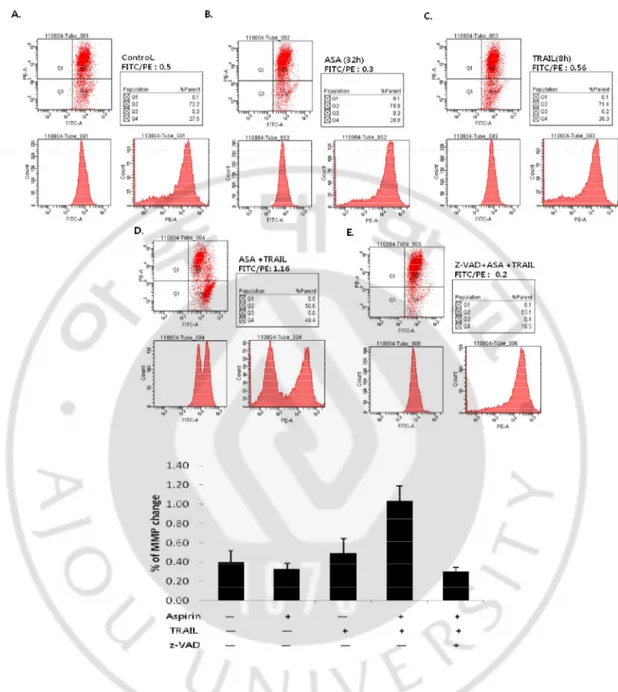
3. Aspirin results in TRAIL-induced caspase-dependent mitochondrial membrane potential change and cytochrome c release
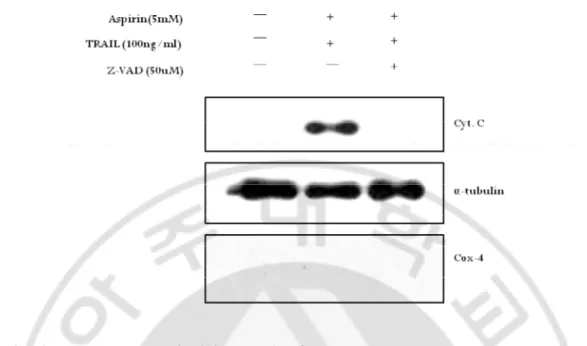
We observed the decrease of Bid and activation of caspase-9 in the combination treatment by western blotting (Fig. 5). These results could be expected that mitochondrial pathway was involved in the combination treatment-induced apoptotic cell death. To confirm this, we checked mitochondrial membrane potential (MMP) change and the release of cytochrome c to cytosol. Additionally, we investigated whether MMP change and the release of cytochrome c were dependent on caspase activities. HeLa cells were incubated with 5mM aspirin for 24hr and followed by 100ng/ml TRAIL treatment for 8hr and analyzed for MMP change using JC-1 dye. As shown in Fig.7D and F, value of FITC/PE was clearly increased in the combination treated cells, indicating that the combined treatment induced loss of MMP (∆Ψm). Furthermore, pre-incubation of zVAD-fmk blocked the loss of ∆Ψm induced by the combination treatment (Fig. 7E), suggesting MMP loss was caspase-dependent. To analyze clearly whether cytochrome c was released to cytosol from mitochondria and it was also caspase-dependent, western blotting was carried out with cytosolic fraction. Cytochrome c was only detected in the combination treatment (Fig. 8). Cytochrome c release can be either dependent or independent on caspase activity (Green, 1998). Therefore, we examined whether zVAD-fmk was capable of preventing the release of cytochrome c. As shown in Fig 8, cytochrome c in cytosolic fraction was completely disappeared by the pre-treatment of zVAD-fmk. These results suggest that the combined treatment of aspirin with TRAIL in HeLa cells promotes mitochondrial pathway of apoptosis depending on caspase activities.
- 17 -
Fig. 7. The combination of aspirin and TRAIL induced MMP change. A.Untreated cells.
B. 5mM aspirin was treated for 32hr. C. 100ng/ml TRAIL was treated for 8hr. D~E. Aspirin was treated for 24hr and followed by TRAIL treatment for 8hr with (D) or without (E) pretreatment of 50uM zVAD-fmk for 1hr. HeLa cells were seeded at 2 х 105 cells per 6-well plate and stabilized for 20hr.
- 18 -
Fig. 8. A pan-caspase inhibitor, zVAD-fmk, prevented cytochrome c release from mitochondria of the combination treated cells. To investigate whether cytochrome c was
released to cytosol from mitochondria and it was caspase-dependent, cytosol fraction was obtained for western blotting analysis. HeLa cells were treated with 5mM aspirin for 24hr followed by 100ng/ml TRAIL for 8hr in the presence or absence of pretreatment of 50uM zVAD-fmk for 1hr. HeLa cells were seeded at 1х106 cells in 60 ф dish and stabilized for 20hr.
- 19 -
4. Aspirin makes TRAIL-resistant HeLa cells TRAIL-sensitive through inhibition of ERK1/2 activation
When aspirin was pretreated at least for 24hr, TRAIL-induced apoptotic cell death of HeLa was considerably enhanced in company with caspase activation. In order to
explore a
major mechanism(s) that
makes HeLa cells TRAIL-sensitive, I examined the changed expressions of various apoptotic regulators by western blotting following aspirin treatment (Fig. 9). It is well known that TRAIL can bind with death receptors, DR4 and DR5, which trigger extrinsic apoptotic signal pathway (Pan et al., 1997). Specially, DR5 levels are up-regulated by chemotherapeutic agents (Jun et al., 2011). Thus, I checked the effect of aspirin on the expression levels of DR4 and DR5. However, the results from western blot analysis showed that aspirin treatment did not alter the total cellular levels of DR4 and DR5 even at 32hr treatment.In addition, changes in the level of anti-apoptotic protein Bcl-2 and
pro-apoptotic protein Bak were negligible. On the other hand, aspirin affected the
activation and expression of ERK1/2 and Bax. The activation of ERK1/2 was
inhibited and
the level of Bax expression was increased by aspirin depending on time of treatment (Fig. 9A). Next, I examined those molecules in the combined treatment of aspirin with TRAIL. Interestingly, I observed obvious decrease of ERK1/2 activation and Mcl-1protein expression, which was up-regulated by TRAIL alone (Fig. 9B). To see whether inactivation of ERK1/2 by aspirin is an important mechanism to make HeLa cells TRAIL-sensitive, we tested the effect of combination treatment of ERK inhibitor PD98059 (20uM) for 2hr and TRAIL (100ng/ml) for 8hr in HeLa cells. The combination of PD98059 and TRAIL enhanced cell death (Fig. 10A). As shown in analysis of annexin V staining, sub G1 population, and MMP change analysis (Fig. 10B, C, and D), apoptotic cell death was enhanced by the combination treatment of PD98059 and TRAIL. As shown in Fig.10E, inhibition of ERK1/2 activation by PD98059 induced caspase activities and down-regulated Mcl-1 level. The results from the treatment of TRAIL and an ERK inhibitor were
similar to those from the treatment of aspirin and TRAIL. It indicates that
ERK1/2 inactivation by aspirin plays a critical role in TRAIL-induced apoptotic cell death through promoted caspase activities.- 20 -
Fig.9. Effect of various time of aspirin treatment and combined treatment of aspirin with TRAIL. A. HeLa cells were treated with 5mM aspirin for 0, 6, 12, 18, 24 and 32hr. B.
The cells were pretreated with 5mM aspirin for 24hr and then treated with 100ng/ml TRAIL for 8hr. Total protein extract was obtained and indicated proteins were analyzed by western blotting with appropriate antibodies. α-tubulin was used as loading control.
- 21 -
A.
- 22 -
E.
Fig.10. Effect of the combined treatment of PD98059 with TRAIL. HeLa cells were
treated with TRAIL (100ng/ml) for 8hr in the presence or absence of PD98059 (20uM) for 2hr. A. Cytotoxicity was analyzed by LDH assay. B. Annexin-V/PI analysis. C. Sub G1 anlaysis. D. MMP change anlaysis. E. Total protein extracts were obtained and analyzed by western blotting. The cells were seeded at 5х103 cells in 96 flate well (A), 1.5 х105 cells in 6-well plate (B,C, and D) or 1х106 cells in 60ф dish (E), respectively and incubated for 20hr.
- 23 -
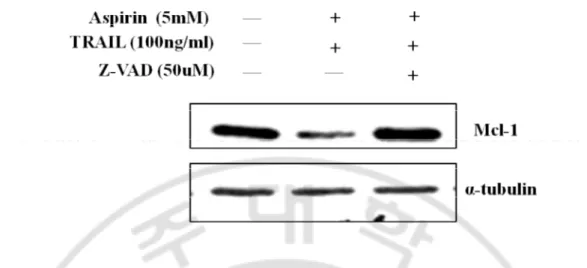
Fig. 11. Effect of caspase about expression of p-ERK and Mcl-1. HeLa cells were
pretreated with 50uM of z-VAD for 1hr and aspirin and TRAIL were treated as previously described. Concentrations of aspirin and TRAIL were 5mM and 100ng/ml, respectively. Cells were harvested and equal amount of protein was processed for western blot analysis to detect target proteins.
- 24 -
Suppl.1. Cytotoxicity was analyzed by LDH assay. HeLa cells were seeded at 2 х 105 cells in 6-well plate and incubated for 20hr. In the combination treatment, aspirin was pretreated for 12hr, 24hr, or at the same time with TRAIL.
- 25 -
Suppl. 2. Morphologies of HeLa cells treated with aspirin, TRAIL or combination of
them in the presence or absence of z-IETD. HeLa cells (2 х 105) were plated onto 6-well
tissue culture plates and stabilized for 20hr. Cells were pretreated with aspirin (5mM) for 24hr and treated continuously with TRAIL (100ng/ml) for 8hr without changing media. For the treatment of z-IETD, the cells were pretreated with z-IETD (50uM) for 1hr and followed by treatment with aspirin, TRAIL or the combination
- 26 -
Ⅳ. DISCUSSION
TRAIL has been shown to be selective in the induction of apoptosis in cancer cells with minimal toxicity to normal tissues. However, not all cancers are sensitive to TRAIL-mediated apoptosis (Pitti et al., 1996). TRAIL-resistance can occurs through dysfunctions of the death receptors DR4 and DR5 (S. Prasad et al., 2011), overexpression of 2 or Bcl-X(L) (Fulda et al., 2002, Langenau et al., 2005), loss of Bax or Bak function (Wang et al., 2001, LeBlanc et al., 2002), high expression of c-FLIP or inhibitor of apoptosis proteins (Irmler et al., 1997, Kim et al., 2008) and reduced release of second mitochondria-derived activator of caspases (Smac/Diablo) from the mitochondria to the cytosol ((Luo et al., 1998). Finally, activation of different subunits of mitogen-activated protein kinases or nuclear factor-kappa B can lead to development of either TRAIL resistance or apoptosis in certain types of cancer cells (Zhang and Fang, 2005). Thus, researchers have attempted to solve a problem of TRAIL-resistance through combimation treatment with other chemical agent. I have tried for the combined treatment of aspirin with TRAIL in TRAIL-resistant HeLa cells. Aspirin has been used as chemopreventive agents of cancers to induce apoptosis or to reduce the incidence of tumor formations in a variety of organs, i.e. colon (Qiao et al., 1998), lung (Hosomi et al.,2000), stomach (Wong et al., 1999), and colorectum (Williams et al., 1997).
In this work, I was interested in by what mechanism HeLa cells are resistant to TRAIL. Interestingly, TRAIL enhanced ERK1/2 activation and increased level of anti-apoptotic protein Mcl-1 in HeLa cells. ERK1/2 has been implicated in the regulation of a variety of cellular processes. However, the precise molecular mechanism of ERK1/2 has still remained controversial. For example, ERK1/2 activation is required in cisplatin-induced apoptosis (Wang. et al., 2000). In contrast, ERK 1/2 activation promotes cell survival in neuronal PC12 cells (She et al., 2000). I observed that TRAIL-mediated ERK activation protects HeLa cells from TRAIL-induced apoptosis through up-regulating Mcl-1, not Bcl-2 (Lee et al. 2006). Different molecules have been reported to play a role in conferring TRAIL resistance in accompany with ERK1/2 activation. For example, the ERK1/2 signaling pathway regulates the expression of Bcl-2, Bcl-XL, and Mcl-1 and promotes the survival of human pancreatic
- 27 -
tumor cells (Boucher et al. 2000). When HeLa cells were treated with TRAIL after pretreatment of aspirin for 24h, more cell death was induced than when they were treated at the same time or pretreated for 12hr (Suppl.1). ERK1/2 inactivation by aspirin affects caspase activiation rather than down-regulation of Mcl-1 level because the level of Mcl-1 was not changed by aspirin. In addition, down-regulated Mcl-1 by the combination treatment was restored by z-VAD (Fig. 11). Although several groups reported down-regulation of Mcl-1 level by aspirin (Park et al.20Mcl-10, Daniel et al. 20Mcl-10), they used the higher concentration (8mM, 10mM) of aspirin, compared with that I used (5mM). Once ERK activation was almost inhibited by aspirin, caspase-3, -8 and -9
were activated and cytochrome c was
released from mitochondria. We observed that a caspase-8 inhibitor, zIETD, blocked
the cell death induced by combined treatment of aspirin with TRAIL (Suppl.2).
These results imply that ERK activation largely suppressed the processing of
caspase-8. Consistently with my results,
there have been several studies to indicate that ERK activation suppresses TRAIL-mediated apoptosis and inhibits the processing of caspase-8 and Bid thereby turning off the mitochondrial amplification loop and that ERK pathway acts as an important modulator of various apoptosis-inducing signals and prevents cell death in different systems including Fas and TRAIL-mediated cell death (Soderstrom et al., 2002, Tran et al., 2001).I also showed that combined treatment of aspirin with TRAIL downregulated level of Mcl-1 protein. In this system, Mcl-1 level was regulated by caspase activation (Fig. 11) as also suggested by Herrant at al. (2004). Downregulated Mcl-1 triggers a mitochondrial pathway and amplifies caspase activation It has been known that the anti-apoptotic Bcl-2 family proteins, including Bcl-2 and Mcl-1, can bind Bid and inhibit Bid's ability to activate Bax or Bak. As a result, the anti-apoptotic Bcl-2 family proteins inhibit apoptosis by sequestering Bid, leading to reduced Bax or Bak activation. Therefore, down-regulation of Mcl-1 can trigger translocation of tBid by caspase-8 from cytosol to the outer mitochondrial membrane (Li at al., 1998). It leads to tBid interacts with pro-apoptotic protein, Bax or Bak leading to the insertion of Bax or Bak into organelle membranes, primarily the outer mitochondrial membrane (Eskes at al., 2000, Clohessy at al., 2006). Activated Bax and/or
- 28 -
Bak form an oligomeric pore in the outer membrane and induce loss of MMP and the release of cytochrome c from mitochondria to cytosol (Desagher at al., 1999). As shown in this study, the released cytochrome c from mitochondria could induce the sequential activation of caspase-9 and caspase-3, which enhance cleavage of Mcl-1 and finally promote apoptosis via mitochondrial pathway in HeLa cells.
In this study, it is suggested that ERK inactivation by aspirin promotes TRAIL-induced caspase activation and activates caspase-dependent cleavage of Mcl-1 which triggers mitochondrial pathway, resulting in enhanced cell death. Taken together, inhibiton of mediated ERK1/2 activation is an important mechanism to overcome TRAIL-resistance in HeLa cells and aspirin plays a role as a ERK 1/2 inhibitor. In conclusion, TRAIL-resistant HeLa cells could be sensitized by pretreatment of aspirin.
- 29 -
REFERENCES
1. Ashkenazi A :Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2: 420-430, 2002
2. Bertagnolli MM: The potential of non-steroidal anti-inflammatory drugs (NSAIDs) for colorectal cancer prevention. J. Surg. Oncol 84: 113–119, 2003
3. Bouralexis S, Findlay DM, Atkins GJ, Labrinidis A, Hay S, and Evdokiou A: Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: resensitisation with chemotherapy. Br. J. Cancer 7: 206–214, 2003
4. Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N.: MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X (L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem 79:355-69, 2000
5. Broaddus VC, Dansen T B, Abayasiriwardana KS, Wilson SM, Finch AJ, Swigart LB, Hunt, AE, and Evan GI: Bid mediates apoptotic synergy between tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and DNA damage. J. Biol. Chem 280: 12486– 12493, 2005
6. Büneker CK, Yu R, Deedigan L, Mohr A, Zwacka RM. IFN-γ combined with targeting of XIAP leads to increased apoptosis-sensitisation of TRAIL resistant pancreatic carcinoma cells.Cancer Lett. Nov 2, 2011
7. Chan AT: Aspirin, non-steroidal anti-inflammatory drugs and colorectal neoplasia: future challenges in chemoprevention. Cancer Causes Control 14: 413–418, 2003
8. Clohessy JG, Zhuang J, de Boer J, Gil-Gomez G, Brady HJM: Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem 281:5750–5759, 2006
- 30 -
9. Daniel IS, M. Pique, M. Barragan et al., “Aspirin induces apoptosis in human leukemia cell independently of NF-κB and MAPKs through alteration of the Mcl-1/Noxa balance,”
Apoptosis, vol. 15, pp. 219–229, 2010
10. Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, and Martinou JC: Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol 144: 891–901, 1999
11. Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA &Goodwin RG. The novel receptor TRAILR4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity, 7(6), 813–820,1997 12. Eskes R, Desagher S, Antonsson B, and Martinou JC: Bid induces the oligomerization
and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol 20:929–935, 2000
13. Fulda S, Jeremias I, and Debatin KM: Cooperation of betulinic acid and TRAIL to induce apoptosis in tumor cells. Oncogene 23: 7611–7620, 2004
14. Green DR: Apoptotic pathways: the roads to ruin. Cell 94: 695-698, 1998
15. Green DR. and Reed, JC :Mitochondria and apoptosis. Science 281: 1309-1311, 1998 16. Gross A., McDonnell, J.M., and Korsmeyer, S.J.: Bcl-2 family members and the
mitochondria in apoptosis. Genes & Dev. 13: 1899-1911, 1999
17. Herrant M, Jacquel A, Marchetti S, Belhacene N, Colosetti P, et al.: Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim-induced apoptosis. Oncogene. 23:7863–7873, 2004
- 31 -
18. Han J, Goldstein LA, Gastman BR, Rabinowich H: Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem 281:10153– 10163, 2006
19. Hosomi Y., Yokose T., Hirose Y., Nakajima R., Nagai K., Nishiwaki Y., and Ochiai A : Increased cyclooxygenase 2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. Lung Cancer 30, 73–81, 2000
20. Jane EP, Premkumar DR and Pollack IF. Bortezomib sensitizes malignant human glioma cells to TRAIL, mediated by inhibition of the NF-{kappa}B signaling pathway. Mol Cancer Ther 10: 198-208, 2011
21. Jun Yang, Yun Yang, Li Tian, Xi-Feng Sheng, Fei Liu, Jian-Guo Cao. Casticin-induced apoptosis involves death receptor 5 upregulation in hepatocellular carcinoma cells. World
J Gastroenterol October 14; 17(38): 4298-4307, 2011
22. Kang MH and Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways incancer therapy. Clin Cancer Res. 15: 1126-1132, 2009
23. Kim S, Lee TJ, Park JW, Kwon TK .Overexpression of cFLIPs inhibits oxaliplatin-mediated apoptosis through enhanced XIAP stability and Akt activation in human renal cancer cells. J Cell Biochem 105:971–979, 2008
24. Langenau DM, Jette C, Berghmans S, Palomero T, Kanki JP, et al. Suppression of apoptosis by bcl-2 overexpression in lymphoid cells of transgenic zebrafish. Blood 105: 3278–3285,2005
25. LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, and Ashkenazi A: Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat.
- 32 -
26. LeBlanc HN, and Ashkenazi A: Apo2L/TRAIL and its death and decoy receptors. Cell
Death Differ 10: 66–75, 2003
27. Lee DY, Lee MW, Lee HJ, Noh YH, Park SC, Lee MY, Kim KY, Lee WB, Kim SS: ERK1/2 activation attenuates TRAIL induced apoptosis through the regulation of mitochondria dependent pathway. Toxicol In Vitro 20: 816-23, 2006
28. Lee Y J, Lee KH, Kim HR., Jessup J M, Seol DW, Kim TH, Billiar TR, and Song, YK: Sodium nitroprusside enhances TRAIL-induced apoptosis via a mitochondria-dependent pathway in human colorectal carcinoma CX-1 cells. Oncogene 20: 1476–1485, 2001 29. Li H, Zhu H, Xu CJ, and Yuan J: Cleavage of BID by caspase 8 mediates the
mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501, 1998
30. Luo X, Budihardjo I, Zou H, Slaughter C, and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490, 1998
31. MacFarlane, M: TRAIL-induced signalling and apoptosis. Toxicol. Lett., 139(2–3), 89– 97, 2003
32. M.Gloria Luciani, Christoph Campregher and Christoph Gasche: Aspirin blocks proliferation in colon cells by inducing a G1 arrest and apoptosis through activation of the checkpoint kinase ATM. Carcinogenesis 10: 2207–2217, 2007
33. Marshall CJ: Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185, 1995
34. Mezosi E, Wang SH, Utsugi S, Bajnok L, Bretz JD, Gauger PG, Thompson NW, Baker Jr JR Interleukin-1β and tumor necrosis factor (TNF)-α sensitize human thyroid epithelial cells to TNF-related apoptosis-inducing ligand-induced apoptosis through
- 33 -
increases in procaspase-7 and bid, and the down-regulation of p44/42 mitogen-activated protein kinase activity. J Clin Endocrinol Metab 89:250–257, 2004
35. Nyormoi O, Mills L, and Bar-Eli M: An MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell
Death Differ 10: 558–569, 2003
36. Pan G, Ni J, Wei YF, Yu G, Gentz R, and Dixit VM: An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277: 815–818, 1997
37. Park SY, Billiar TR, and Seol DW: IFN-gamma inhibition of TRAIL-induced IAP-2 upregulation, a possible mechanism of IFN-gamma-enhanced TRAIL-induced apoptosis.
Biochem. Biophys. Res. Commun 291: 233–236, 2002
38. Park IS, Jo JR, Hong H, and Jang BC et al: Aspirin induces apoptosis in YD-8 human oral squamous carcinoma cells through activation of caspases, down-regulation of Mcl-1, and inactivation of ERK-1/2 and AKT. Toxicology in Vitro 24 713–720, 2010
39. Pitti RM, Marsters SA, Ruppert S, et al.: Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271: 12687–12690, 1996
40. Qiao L, Hanif R., Sphicas E., Shiff SJ, and Rigas B.: Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem. Pharmacol. 55,53–64, 1998
41. S. Prasad, V. R. Yadav, R. Kannappan, and B. B. Aggarwal, Ursolic acid, a pentacyclin triterpene, potentiates TRAILinduced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK, The
- 34 -
42. Sale EM, Atkinson PG, Sale GJ: Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J 14: 674–684, 1995
43. Seger R and Krebs EG: The MAPK signaling cascade. Faseb J 9: 726–735, 1995 44. She, Q.B., Chen, N., Dong, Z. : ERKs and p38 kinase phosphorylate p53 protein at serine
15 in response to UV radiation. J. Biol. Chem. 275, 20444–20449 , 2000
45. Shuanglin Xiang, Zhenhua Sun, Qiongzhi He, Feng Yan, Yijun Wang and Jian Zhang: Aspirin inhibits ErbB2 to induce apoptosis in cervical cancer cells. Med Oncol 379–387, 2010
46. Soderstrom TS, Poukkula M., Holmstrom TH., Heiskanen KM, & Eriksson JE :Mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in :Mitogen-activated Tcells abrogates TRAIL-induced apoptosis upstream of the mitochondrial amplification loop and caspase-8. J. Immunol., 169(6),2851–2860, 2002
47. Strasser A., O'Connor L., and Dixit VM : Apoptosis signaling. Annu. Rev. Biochem 69:217-245, 2000
48. Thiyam Ramsing Singh, Sharmila Shankar, Xufen Chen, Mohammed Asim, and Rakesh K. Srivastava: Synergistic Interactions of Chemotherapeutic Drugs and Tumor Necrosis Factorrelated Apoptosis-inducing Ligand/Apo-2 Ligand on Apoptosis and on Regression of Breast Carcinoma in Vivo. Cancer Res 63: 5390-5400, 2003
49. Tillman DM, Izeradjene K, Szucs KS, Douglas L, and Houghton JA: Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer
- 35 -
50. Tran SE, Holmstrom TH, Ahonen M, Kahari VM, & Eriksso JE :MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J. Biol. Chem., 276(19), 16484–16490, 2001
51. Wang X, Martindale JL, Holbrook NJ: Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem 275:39435-39443, 2000
52. Wang X : The expanding role of mitochondria in apoptosis. Genes & Dev. 15: 2922-2933, 2001
53. Williams CS, Smalley W, and DuBois RN.: Aspirin use and potential mechanisms for colorectal cancer prevention. J. Clin. Investig. 100, 1325–1329, 1997
54. Wong BC., Zhu GH., and Lam SK.: Aspirin induced apoptosis in gastric cancer cells.
Biomed. Pharmacother. 53, 315–318, 1999
55. Zhang L, Fang B: Mechanisms of resistance to TRAIL-induced apoptosis in cancer.
- 36 -
- 국문요약 -
Aspririn 과 TRAIL 병합 처리시 자궁 경부암인 HeLa cell 에서
일어나는 효과에 관한 분석
아주대학교 대학원 의생명학과 임 세 란
(지도교수 : 장 영 주)
TRAIL(TNF-related apoptosis inducing ligand)는 세포 표면의 death receptor 들과 결합하여 세포질내 death domain 을 통해 caspase 을 활성화 시키는 방법으로 세포사멸을 유도한다. 이런 TRAIL 은 암세포에만 선별적으로 세포사멸을 유도하기 때문에 각광받는 물질이기도 하다. 그러나 TRAIL 에 저항성을 보이는 암세포들이 있어 이들 치료를 위해 저농도의 항암물질과 병합처리하는 방법이 사용되어지고 있다. 여기서 우리는 TRAIL 의 다른측면에 관점을 두고 실험에 임했다. 즉 TRAIL 은 암세포에 죽음의 신호뿐 아니라 생존의 신호로도 작용한다는 것이다. 그로인해 암세포가 TRAIL 에 저항성을
- 37 -
보이는 것이라 생각했고 이런 생존신호를 억제시킴으로써 TRAIL 에 민감해질 것이라 생각했다. 우리는 TRAIL 의 생존신호를 억제시키는 물질로 aspirin 을 사용했으며, 이것은 TRAIL 에 저항성을 보이는 자궁경부암세포인 HeLa cell 의 세포사멸을 향상시켰다.
Aspirin 은 HeLa cell 의 증식을 억제시키지만 세포독성이 나타나지는 않았고, TRAIL 은 약간의 세포사멸을 유도했으나, 그 농도가 증가한다고 해서 더 많은 사멸을 유도하지는 않음을 LDH 와 MTT 방법을 통해 알 수 있었다. 하지만 aspirin 을 24 시간 선처리 후 TRAIL 을 8 시간 처리했을때, 세포는 급격히 죽음에 이르렀고, 우리는 annexin-V-FITC 와 PI 염색을 하여 유세포 분석기을 통해 이들 죽음이 괴사가 아닌 세포사멸임을 알았다. 또한 세포내 어떤 단백질의 변화가 있는지 알아보고 그 기작을 규명하기 위해 western blot 을 실행했다. Aspirin 단독에서는 세포증식을 촉진시키는 p-ERK 의 감소가 눈에 띄였고, 이와는 반대로 TRAIL 에서는 p-ERK 가 증가하면서 Mcl-1 도 증가했다. 우리는 TRAIL 에 의해서 p-ERK 가 증가하고 이것이 세포사멸을 억제하는 단백질인 Mcl-1 을 증가시켜서 TRAIL 에 저항성을 가지도록 한다고 생각했다. 그래서 aspirin 과 TRAIL 을 병합처리 했을 때 p-ERK 가 감소하면서 그에 따라 Mcl-1 도 감소해서 세포사멸이 증가한다고 생각했으나, caspase inhibitor 인 z-VAD 을 처리하여 본 결과 Mcl-1 은 활성화된 caspase 에 의하여 감소한 것으로 보인다.
- 38 -
결론적으로, TRAIL 에 의해 증가되는 p-ERK 는 aspirin 선처리에 의해 억제되고, 이것이 TRAIL 에 의한 caspase 의 활성을 향상시켜 Mcl-1 을 감소시킨다. 이것은 tBid 의 미토콘드리아 외막으로의 이동을 용이하게 하여 Bax 또는 Bak 이 미토콘드리아 외막에 구멍을 만들도록 한다. 이런 세포내 사멸유도 경유의 활성화는 caspase 의 활성을 더욱 더 향상시켜 세포사멸이 짧은 시간에 이루어 지도록 하는 것 같다. 이 논문에서 우리는 aspirin 이 TRAIL 의 저항성을 억제하여 세포사멸을 향상시키는데 유용한 물질임을 제시한다.