저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Master's Thesis in Molecular Medicine
The role of IFITM1 in malignant
progression of neurofibromas in NF1
Ajou University Graduate School
Major in Molecular Medicine
Department of Biomedical Sciences
The role of IFITM1 in malignant
progression of neurofibromas in NF1
Hyon J. Kim, Advisor
Seon-Yong Jeong, Advisor
I submit this thesis as the
Master's thesis in Molecular Medicine.
August 2017
Ajou University Graduate School
Major in Molecular Medicine
Department of Biomedical Sciences
i Abstract
The role of IFITM1 in malignant progression of
neurofibromas in NF1
Neurofibromatosis type 1 (NF1) is one of the most commonly inherited autosomal dominant human genetic disorder with an incidence of approximately 1 in 3000~3500 individuals worldwide. NF1 is caused by loss-of-function mutations in the NF1 gene encoding neurofibromin, a GTPase-activating protein. Because the pathogenesis of the tumor progression of benign plexiform neurofibromas (PNs) to malignant peripheral nerve sheath tumors (MPNSTs) remained unclear, genetic and epigenetic changes involved in MPNST pathogenesis. Here I found that interferon-induced transmembrane protein 1 (IFITM1) was downregulated in MPNST tissues compared to that in PN tissues from NF1 patients by immunohistological staining and/or Western blot analysis. Overexpression of IFITM1 in the NF1-deficient MPNST tumor cells resulted in a decrease in Ras activation (GTP-Ras), while downregulation of IFITM1 by treatment of small interfering RNA in normal-phenotypic NF1-deficient cells caused an increase in Erk1/2 activation (phosphorylated Erk1/2), indicating that expression level of IFITM1 is closely related with tumor progression in NF1. Treatment of interferon-γ (INF-γ) in the MPNST cells caused elevated expression of IFITM1, thereby leading to a decrease in the Ras activation and its downstream Erk1/2 activation. Notably, INF-γ produced a sensitization effect on enhancing cytotoxicity of MPNST cells by cotreatment of low-dose cisplatin and gemcitabine. These results provide a new potential target for chemotherapy in the NF1 patients with MPNSTs.
--- Keywords: neurofibromatosis type 1 (NF1), malignant peripheral nerve sheath tumor (MPNST), tumor progression, IFITM1, Ras, Erk1/2, interferon-γ
ii
TABLE OF CONTENTS
ABSTRACT ... ⅰ
TABLE OF CONTENTS ... ii
LIST OF FIGURES ... iv
LIST OF TABLES ... vii
I. INTRODUCTION ... 1
II. MATERIALS AND METHODS ... 7
A. Tumor tissue samples ... 7
B. Primary tissue-cultured cells and cell lines ... 7
C. Hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) ... 8
D. Plasmid constructs and small interfering RNAs (siRNAs) ... 9
E. Reverse transcription-PCR (RT-PCR)... 10
F. Western blot analysis ... 10
G. Ras activation assay ... 11
H. Cell viability assay ... 12
III. RESULTS ... 14
A. Downregulation of IFITM1 in MPNST tissues of patients with NF1 ... 14
B. Downregulation of IFITM1 in MPNST cells from NF1 patients and MPNST cell lines ... 17
C. Correlation of IFITM1 expression and Ras activation levels in primary MPNST cells and MPNST cell lines... 19
iii
D. Effects of interferon-γ on enhancing IFITM1 expression and reducing Ras
and Erk1/2 activation in MPNST cells ... 21
E. Sensitization effects of interferon-gamma on enhancing cytotoxicity of the MPNST cells co-treated with cisplatin and gemcitabine ... 23
IV. DISCUSSION ... 26
V. CONCLUSION ... 29
REFERENCES... 30
iv
LIST OF FIGURES
Figure 1. The various phenotypes of neurofibromatosis type 1. ... 2 Figure 2. Representation of NF1 interactions with the Ras and PI3K pathways...6 Figure 3. Immunohistochemical staining of IFITM1 in the plexiform neurofibroma
(PN) and malignant peripheral nerve sheet tumor (MPNST) tissues from patients with NF1 ... 16 Figure 4. Comparison analyses of the gene expression level of IFITM1 in primary
NF1-deficient cells and MPNST cell lines. ... 18
Figure 5. IFITM1 level-dependent alterations of Ras and Erk1/2 activation in NF1-deficient primary cells and MPNST cell lines. ... 20 Figure 6. Effects of interferon-gamma treatment on IFITM1 expression and Ras
activation levels in the MPNST cell lines. ... 22 Figure 7. Effects of interferon-gamma on the sensitization of anticancer drugs in
the MPNST cell lines. ... 24 Figure 8. Sensitization effects of interferon-gamma on enhancing cytotoxicity of
v
LIST OF TABLES
1
I. Introduction
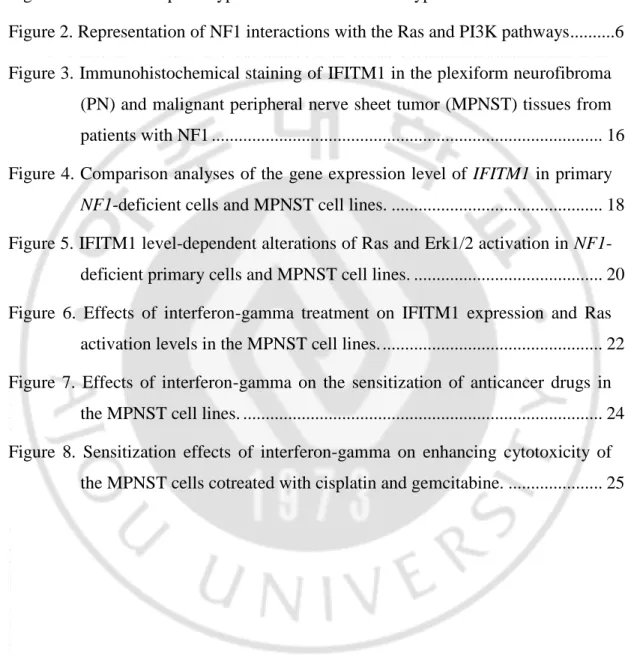
Neurofibromatosis type 1 (NF1; OMIM #162200) is a commonly inherited autosomal dominant disorder, occurring with an incidence of 1 in 3,000~3,500 individuals worldwide (McClatchey, 2007; Boyd et al., 2009). NF1 is caused by loss of function mutations in the NF1 gene (GenBank gene ID: NG_009018.1), which encodes a GTPase-activating protein, neurofibromin (Cawthon et al., 1990). The major clinical features of NF1 include cafe-au-lait spots, freckling of the axillary or inguinal region, Lisch nodules, optic nerve glioma, bone dysplasias, and nerve sheath tumors in the peripheral nervous system, including benign cutaneous neurofibromas, benign plexiform neurofibromas (PNs), and malignant peripheral nerve sheet tumors (MPNSTs) (Jett and Friedman, 2010) (Figure 1).
Malignant peripheral nerve sheath tumors (MPNSTs), a type of aggressive sarcomas, are a major cause of mortality in patients with NF1 (Grobmyer et al., 2008; Brems et al., 2009; Katz et al., 2009; Spurlock et al., 2010). A lifetime risk of developing MPNSTs in NF1 patients has been estimated at 8-13% (Evans et al., 2002) and 5.9-10.3% (McCaughan et al., 2007). The majority of NF1-associated MPNSTs (about ~85% of cases) are high-grade malignant tumors. Malignant transformation of benign PNs to MPNSTs in NF1 is notable (Tucker et al., 2005) and is of far greater concern to NF1 patients (McQueen et al., 2008), but its pathogenesis is poorly understood.
2
Figure 1. The various phenotypes of neurofibromatosis type 1. (A) Café-au-lait spots, (B) neurofibroma, (C) Lisch nodules, (D) plexiform neurofibroma, (E) Scoliosis, (F) tibial dysplasia, (G) optic pathway glioma, and (H) pontine glioma.
3
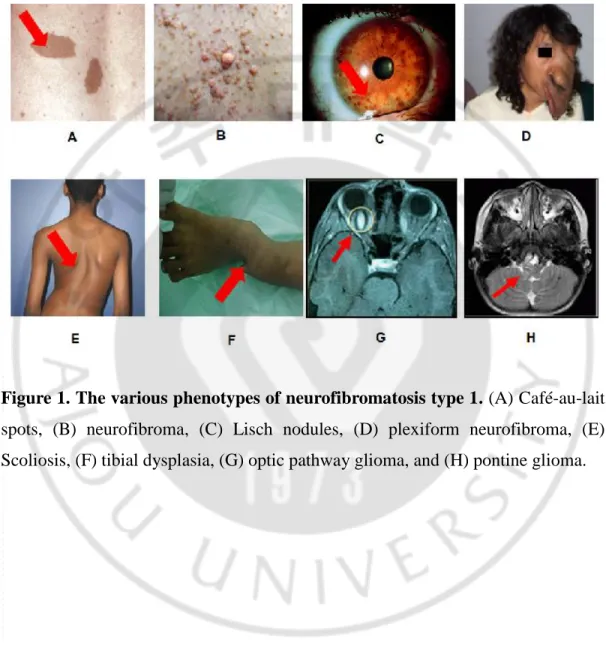
Since neurofibromin is a major Ras inactivator and plays a role as a tumor suppressor, the lack of neurofibomin resulting from NF1 mutation causes disruptions in the RAS-mitogen-activated protein kinase (MAPK) and PI3K-AKT-mTOR signaling pathways, which is implicated in the tumorigenesis and tumor progression of PN to MPNST (Le and Parada, 2007; Katz et al., 2009; Rebecca and Smalley, 2014) (Figure 2).
MPNST, also called malignant schwannoma or neurofibrosarcoma, develops in 8–13% of NF1 patients (Evans et al., 2002), and it represents a major cause of mortality in NF1 patients (Grobmyer et al., 2008). Thus, the pathogenesis of the malignant transformation of PN to MPNST in NF1 patients has attracted great attention (Brems et al., 2009; Thway and Fisher, 2014). However, the exact molecular mechanisms of MPNST pathogenesis remain unclear. Neurofibromas are composed of mixture of cell types including Schwann cells, fibroblast cells, mast cells and perineural cells (Gottfried et al., 2006). Schwann cells are believed to be the primary pathogenic cell source in neurofibromas (Carroll and Stonecypher, 2005). Since complete loss of NF1 gene has been identified in exclusively Schwann cells of neurofibromas (Kluwe et al., 1999a; Rutkowski et al., 2000; Serra et al., 2000) and loss of NF1 in the Schwann cell lineage is sufficient to generate
tumors in mice (Zhu et al., 2002), bi-allelic inactivation of both NF1 alleles (NF1-/-)
in Schwann cells by germline NF1 mutation at one allele and additional somatic loss of heterozygosity (LOH) at the remaining functional NF1 locus has been
4
suggested to be a major cause of the NF1 tumorigenesis. In addition,
haploinsufficiency in other types of cells (NF1+/-) in neural crest-derived tissues
including fibroblast cells, mast cell and perineurial cells is also known to play a important role in pathoetiology of NF1 (Gottfried et al., 2006; Staser et al., 2010; Jouhilahti et al., 2011).
Bi-allelic loss of NF1 gene function (NF1-/-) due to the somatic LOH of the NF1
gene has been reported to be essential for MPNST development (Upadhyaya et al., 2008). However, LOH at the NF1 locus was also found in PNs (Kluwe et al., 1999b), suggesting that other genetic and/or epigenetic alterations may be involved in tumor progression in NF1. Emerging evidence has suggested that additional driver gene mutations and/or expression alterations contribute to the tumor progression of PNs to MPNSTs (Katz et al., 2009). Mutations in tumor suppressor genes, including TP53, CDKN2A, and RB, are commonly identified in MPNSTs (Legius et al., 1994; Nielsen et al., 1999; Mawrin et al., 2002). It was also reported that several cell-cycle and signaling regulation genes, including CDKN2A, CD44,
CXCR4, EGFR, HGF, MET, PDGFR, PDGFRA, RB1, SOX9, and TP53, are
deregulated in MPNSTs (Upadhyaya, 2011; Mo et al., 2013).
In addition, many differentially expressed genes between benign neurofibromas and MPNSTs, such as KIT, ERBB2, MET, TGFB1, HGF, TNXB, TNC, MTOR,
TSC2, PTEN, BCL2L1, CDK4, FOXM1, BUB1B, PBK, and NEK2, have been
5
microarray-based, quantitative reverse transcription PCR-based, or comparative genomic hybridization array-based analyses (Watanabe et al., 2001; Johannessen et al., 2005; Levy et al., 2007; Carroll and Ratner, 2008; Gregorian et al., 2009; Yu et al., 2011; Park et al., 2013; Stricker et al., 2013). Based on the analysis of the RT-PCR-based differential display data of the normal phenotype, benign PN, and MPNST tissues of an NF1 patient, previous study found that IFITM1 is significantly upregulated in MPNST tissues (Jeong et al., 2008).
Here, I report a IFITM1 gene encoding an IFITM1 protein as a novel candidate that plays a critical role in tumor progression of PN to MPNST in NF1. IFITM1 was downregulated in MPNST tissues and cells derived from NF1 patients. Manipulation of IFITM1 expression in the NF1-deficient cells demonstrated the key role of IFITM1 in MPNST pathogenesis. This finding may provide insight into the underlying mechanisms of tumor progression in NF1.
6
Figure 2. Representation of NF1 interactions with the Ras and PI3K pathways (Le and Parada, 2007). NF1 constrains Ras activity in the normal cell. Therefore, loss of NF1 expression leads to elevated Ras activity, dysregulated cell growth and tumorigenesis. Ras is activated at the plasma membrane upon binding of growth factor receptors to specific ligands, triggering the recruitment of a complex containing the adapter protein growth factor receptor bound protein 2 (Grb2) and the Ras guanine nucleotide exchange factor SOS to the site of receptor tyrosine kinase activation. Here, Ras is catalyzed to switch to its GTP-bound state. This active form of Ras then binds and activates the kinase Raf and phosphatidylinositol 3'-kinase (PI3K), which then sets off a kinase cascade, culminating the activation of mitogen-activated protein kinases (MAPK)/MAPKK and PI3K pathways
7
II. Materials and Methods
A. Tumor tissue samples.
I used the tumor tissues of seven patients diagnosed with NF1 at the Ajou University Hospital according to NF1 diagnostic criteria (Ferner et al., 2007) (Table 1). The PN and MPNST tumor samples were obtained through surgical resection at the Ajou University Hospital. In the case of patients P6 and P7, two tumor specimens obtained from surgeries at two different times were used in this study. The study was approved by the Institutional Review Board Committee of the Ajou University School of Medicine.
B. Primary tissue-cultured cells and cell lines.
I used three types of previously established primary tissue-cultured NF1-deficient cells: normal phenotype tissues (PC-N) and the MPNST tissues (PC-M) of NF1 patient P7 (Lee et al., 2012). The cellular characteristics, including GTP-Ras activity and its downstream effectors in the three cell types, were previously demonstrated (Lee et al., 2012). Cells were grown in DMEM media (Hyclone
Laboratories, Logan, UT, USA) supplemented with 15% FBS (Hyclone
Laboratories), penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were used from passages 5 through 10. The human normal Schwann cells (HSCs) and
8
purchased from the American Type Culture Collection (Manassas, VA, USA) and grown in DMEM media supplemented with 10% FBS. All cultured cells were
incubated at 37°C in a humidified atmosphere containing 5% CO2.
C. Hematoxylin and eosin (H&E) staining and immuno-histochemistry (IHC).
The tumor tissue specimens from eight patients with NF1were formalin-fixed and embedded in paraffin wax for pathological evaluation by H&E staining and IHC. Serial 3 μm sections were prepared on glass using a cryostat, and the slides were stained with H&E. For IHC, the blocks were cut at 10 μm thickness and adhered to glass slides. They were deparaffinized in xylene and rehydrated with graded ethanol, which was followed by antigen retrieval in boiling 10 mM citrate buffer (pH 6.0) for 4 min. Immunostaining was carried out using the UltraVision
LP detection system and the HRP Polymer & DABPlus Chromogen (Thermo
Scientific, Carlsbad, CA, USA) according to the manufacturer’s instructions. Briefly, the sections were incubated with Ultra V Block for 5 min at room temperature to reduce the nonspecific background, and they were then treated with hydrogen peroxide to block endogenous peroxidase activity. The sections were incubated with the primary antibody for 1 h at room temperature and then incubated with HRP polymer for 20 min at room temperature. The reaction product was visualized with the DAB Chromogen.Pathological evaluation was performed under light microscopy. Anti-IFITM1 and anti-S100 antibodies were purchased
9
from Boster Biological Technology (Wuhan, China) and Thermo Scientific (Rockford, IL, USA), respectively.
D. Plasmid constructs and small interfering RNAs (siRNAs).
Full-length human IFITM1 cDNA (GenBank: NM_003641) was amplified by reverse transcription polymerase chain reaction (RT-PCR) using the primers
5′-TTCTCGAGCTATGCACAAGGAGGAACATGAGGTGG-3′ (the sequences
underlined indicate XhoI restriction enzyme site) and 5′-CTGGATCCAGCATTGC ACAGTGGAGTGCA-3′ (the sequences underlined indicate BamHI restriction enzyme site), which were from the total RNAs of the human skin tissue–cultured fibroblast cells. The cDNAs were subcloned into the pcDNA3.1(-) vector (Clontech, Palo Alto, CA, USA) using the XhoI and BamHI restriction enzyme sites.
The siRNAs were synthesized by Genolution Pharmaceuticals, Inc. (Seoul, South Korea). The target sequences for the siRNAs were as follows: 5′-AACTCATGACCATTGGATTCA-3′ for the IFITM1 gene and 5′-CCTACGCCAC CAATTTCGT-3′ for the nonspecific scramble siRNA control. Cell transfection of the siRNAs and plasmid constructs was conducted using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) and Lipofectamine 2000 (Invitrogen). Cells were transfected with either 50 nM each Bcl-xL siRNA or nonspecific control siRNA and then incubated for 72 h. Cells were transfected with IFITM1 plasmid construct or an empty pcDNA3.1 vector and then incubated for 24 h. Cells were
10 harvested and lysed in the proper lysis buffer.
E. Reverse transcription-PCR (RT-PCR).
Total RNAs were isolated from the cultured cells using TRIzol reagent (Invitrogen), and they were treated with RNase-free DNase I (Invitrogen) to avoid amplification of the genomic DNA and were subsequently reverse transcribed using the RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas,
Burlington, ON, Canada) with the oligo(dT)15–18 primer. PCR amplification was
done using the Ex-Taq DNA polymerase (TaKaRa, Shiga, Japan) at an annealing temperature of 60°C for 25 cycles. The gene specific primers used were as follows: 5′-CTGGTGCTGTCTGGGCTTCATAGC-3′ and 5′-GGTCATGAGGATGCCCA GAATCAGG-3′ for the IFITM1 gene and 5′-TGTTGCCATCAATGACCCCTT-3′ and 5′-CTCCACGACGTACTCAGCG-3′ for the GAPDH gene (control).
F. Western blot analysis.
Cultured cells were lysed in RIPA buffer [150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)], and 50 mM Tris buffer, pH 8.0). The proteins were heated at 100°C for 10 min and analyzed by SDS-polyacrylamide gel electrophoresis on 8–12% SDS-polyacrylamide gels. The proteins were electroblotted onto PVDF membranes (Millipore, Milford, MA, USA). The membrane blots were blocked with 5% (w/v) nonfat dried milk, incubated with
11
primary and secondary antibodies, and then visualized with the Enhanced Chemiluminescence Western Blotting Detection System (WEST-ZOL plus; iNtRON Biotechnology, Daejeon, Korea). Anti-extracellular regulated kinase (Erk)1/2 and anti-phosphorylated Erk1/2 antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-IFITM1 was purchased from Boster Biological Technology, and anti-α-Tubulin, HRP-conjugated goat anti-rabbit IgG, and HRP-conjugated goat anti-mouse IgG antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
G. Ras activation assay.
Ras activation was assayed using the Ras activation assay kit (Upstate Biotechnology, Lake Placid, NY, USA). Briefly, cells were lysed in lysis buffer (25
mM HEPES pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM
EDTA, 2% glycerol, 25 mM NaF, and 1 mM Na3VO4) on ice and centrifuged at
14,000 × g for 5 min at 4°C. A total of 300 μg cellular lysates were incubated with Raf-RBD agarose beads at 4°C for 1 h. The beads were washed 3 times with 1 ml of ice-cold lysis buffer, resuspended in 2×Laemmli sample buffer, boiled, and separated on SDS-PAGE gels, which was followed by Western blot analysis using the anti-Ras and anti-phosphorylated Ras antibodies that are contained in the kit.
12 H. Cell viability assay.
Cell viability assay was performed by using D-Plus™ CCK cell viability assay kit (DonglinLS, Korea). The cultured cells were plated at a density of 7×10³ cells in 96-well plate, incubated overnight, and were treated with the indicated concentration of drugs. After 24 h and 48h incubations, 10 μl of CCK reagent was added, and the cells were incubated for another 2 h and then absorbance was measured at a wavelength of 450 nm with an ELISA microplate reader (Bio-Rad Model 680).
14
III. Results
A. Downregulation of IFITM1 in MPNST tissues of patients with NF1
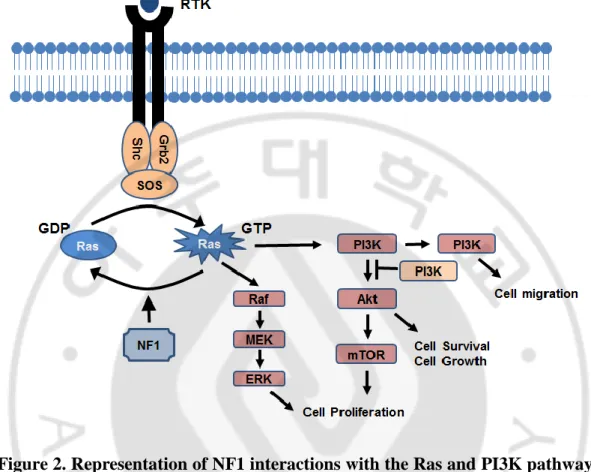
Previous report found that IFITM1 is significantly upregulated in MPNST tissues (Jeong et al., 2008). I attempt to confirm this finding in more tumor tissues of NF1 patients using immunohistochemical (IHC) analysis. The clinical features and genotypes of the seven NF1 patients analyzed in this study are summarized in Table 1. First, the PN and MPNST tumor specimens of seven NF1 patients were evaluated using histopathological analysis with H&E staining (Table 1). Since Schwann cells are the primary pathogenic cell source in PN and MPNST tumors, I further evaluated tumor specimens through IHC analysis using the Schwann cell lineage marker S100 (Table 1).
I compared the IFITM1 protein levels between the PN and MPNST tumor tissues using IHC analysis. The expression levels of IFITM1 were higher in the MPNST tissues (P4–P5) than in PN tissues (P1–P3) (Figure 3A). Next, to investigate whether the IFITM1 expression level was altered in accordance with tumor progression, I compared the IFITM1 levels in the two tumor tissues resected at two different times from the same patient. In patient P6, different IFITM1 levels were observed in two PN tumors with a five-year interval: the latter showed lower
15
from the first surgery and the MPNST tumor from the second surgery (a two-year interval) and found significantly lower IFITM1 expression in the MPNST tumor than in the PN tumor (Figure 3B).
16
Figure 3. Immunohistochemical staining of IFITM1 in the plexiform neurofibroma (PN) and malignant peripheral nerve sheet tumor (MPNST) tissues from patients with NF1. (A) Comparison of IFITM1 protein levels between tumor tissue sections of PNs (patients P1–P3) and MPNSTs (patients P4– P5). (B) Comparison of IFITM1 protein levels in tumor tissue sections from the same patient that were taken at two different times. Immunohistological analysis of IFITM1 was carried out on PN and MPNST tissue sections.
17
B. Downregulation of IFITM1 in MPNST cells from NF1 patients and MPNST
cell lines
Next, I confirmed this result in the primary NF1-deficient cells from NF1 patient P7 (Lee et al., 2012): normal-phenotypic cells (PC-N) and MPNST cells (PC-M),
and two Schwann-like MPNST cell lines, sNF02.2 (NF1+/-) and sNF96.2 (NF1-/-)
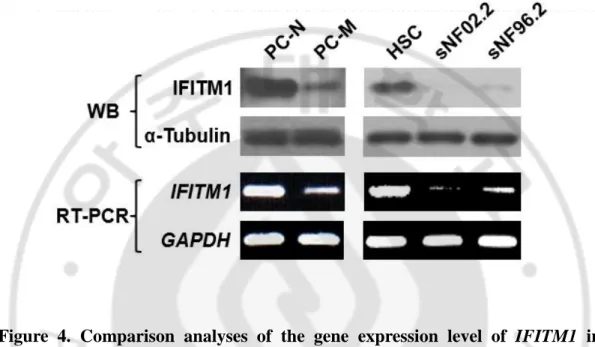
(Li et al., 2004), that express a low level of IFITM1. Western blot analysis revealed that IFITM1 expression was downregulated in malignant MPNST cells, PC-M, sNF02.2 and sNF96.2 (Figure 4).
To determine whether the reduced expression of IFITM1 in MPNST cells was responsible for the changes in the transcriptional expression of the IFITM1 gene,
IFITM1 mRNA levels were examined by RT-PCR. Consistent with the results for
the protein level, the mRNA expression level of IFITM1 also downregulated in MPNST cells (Figure 4).
18
Figure 4. Comparison analyses of the gene expression level of IFITM1 in primary NF1-deficient cells and MPNST cell lines. Primary-cultured normal-phenotypic cells (PC-N), and MPNST cells (PC-M) from NF1 patient P7 and two MPNST cell lines with NF1 mutations were used in this study. Protein levels of IFITM1 and mRNA level of IFITM1 in the normal and MPNST cells were assessed by Western blotting and real-time reverse transcription polymerase chain reaction (RT-PCR), respectively. α-tubulin protein level and GAPDH mRNA level were used as the internal controls.
19
C. Correlation of IFITM1 expression and Ras activation levels in primary
MPNST cells and MPNST cell lines
To examine the functional role of IFITM1 in MPNST pathogenesis, I manipulated the IFITM1 gene expression and investigated if the Ras signaling was altered according to the IFITM1 expression level in the normal cells and malignant tumor cells.
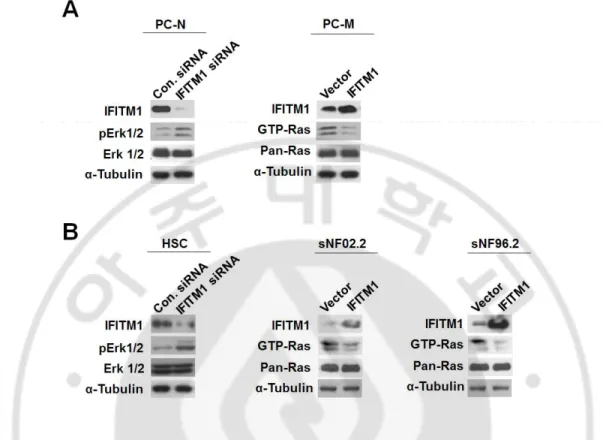
Downregulation of IFITM1 expression in the primary PC-N cells through the treatment of the siRNAs resulted in an increase Erk1/2 activation (phospholylated Erk1/2, pErk1/2 (Figure 5A). In contrast, overexpression of IFITM1 in the primary PC-M cells caused a decrease in RAS activation (Figure 5A).
Next, I carried out the same experiment using the cell lines. Knockdown of IFITM1 in normal human Schwann cells (HSC) by treatment of the siRNAs resulted in an increase ERK1/2 activation. When IFITM1 was overexpressed in two MPNST cell lines, sNF02.2 and sNF96.2, both cell lines showed decreased GTP-Ras, as in the primary PC-M cells (Figure 5B). These results indicate that the IFITM1 level is closely involved in the Ras/Raf/Mek/Erk signaling pathway.
20
Figure 5. IFITM1 level-dependent alterations of Ras and Erk1/2 activation in
NF1-deficient primary cells and MPNST cell lines. (A) Knockdown of IFITM1
using small interfering RNAs (siRNAs) in primary-cultured normal-phenotypic cells (PC-N) from NF1 patient P7. PC-N cells were treated with IFITM1 siRNAs (50 nM) or nonspecific scramble control siRNAs (50 nM) and incubated for 72 h. Overexpression of IFITM1 in primary-cultured MPNST cells (PC-M) from NF1 patient P7. PC-M cells was transfected with a pCDNA3.1 plasmid vector or a
IFITM1 construct and then incubated for 24 h. (B) Knockdown of IFITM1 using
siRNAs in normal human Schwann cells (HSC). Cells were treated with IFITM1 siRNAs (50 nM) or nonspecific scramble control siRNAs (50 nM) and incubated for 72 h. Overexpression of IFITM1 in sNF02.2, sNF96.2 by transfection of a
IFITM1 plasmid construct and incubated for 24 h. Cells were lysed in RIPA buffer
21
D. Effects of interferon-γ on enhancing IFITM1 expression and reducing Ras
and Erk1/2 activation in MPNST cells
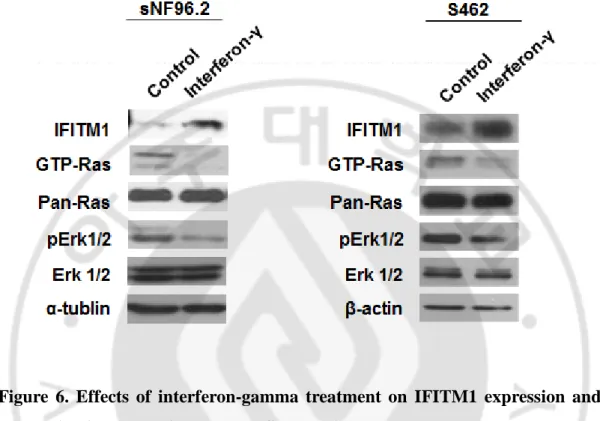
Next, I investigated the effects of interferon-γ (INF-γ) on the IFITM1 expression and further the Ras/Raf/Mek/Erk signaling pathway of MPNST cells. Treatment of INF-γ in MPNST sNF96.2 and S462 cells resulted in a significant increase of IFITM1 in both cells. In addition, the activated form of Ras GTPase (GTP-Ras) and its downstream effector, the activated forms of Erk1/2 (phosphorylated Erk1/2) were dramatically reduced in both MPNST cells by treatment of INF-γ (Figure 6).
These results indicate that INF-γ treatment sufficiently induce IFITM1 expression and IFITM1 is closely engaged in the Ras signaling pathway in MPNST cells.
22
Figure 6. Effects of interferon-gamma treatment on IFITM1 expression and Ras activation levels in the MPNST cell lines. sNF96.2 and S462 cells were treated with interferon-γ (1,000 U/ml) for 12 h and lysed in the appropriate buffers. GTP-Ras was precipitated with beads immobilized with GST fusion protein containing the Ras-binding domain of Raf. Protein levels of IFITM1, GTP-Ras, Pan-Ras, phosphorylated Erk1/2, Erk-1/2, α-Tubulin and β-actin were analyzed by immunoblotting, as indicated.
23
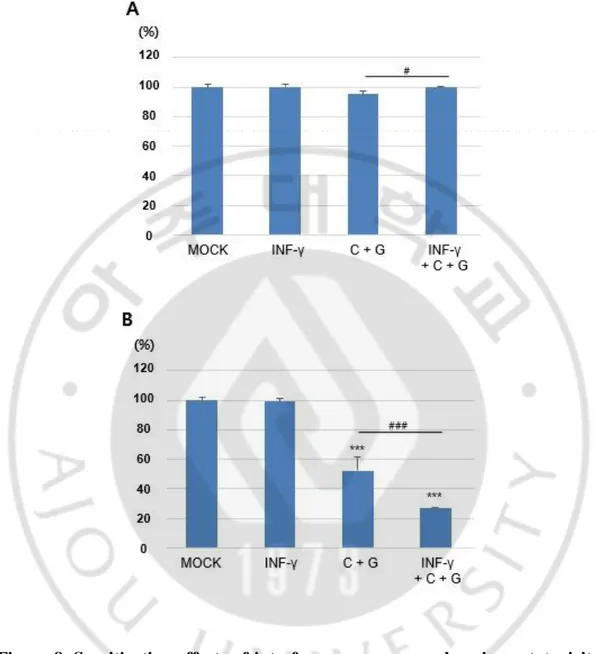
E. Sensitization effects of interferon-gamma on enhancing cytotoxicity of the
MPNST cells co-treated with cisplatin and gemcitabine.
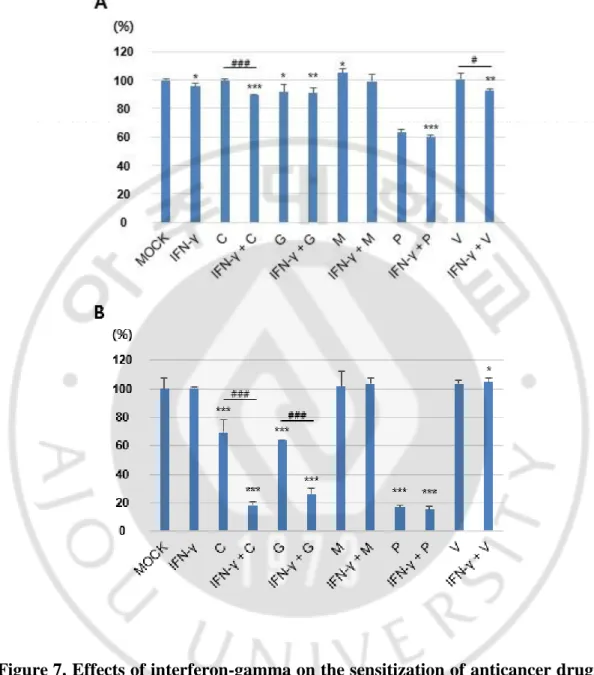
I examined whether co-treatment with INF-γ produced and anticancer drugs enhance the cytotoxicity of MPNST cells. INF-γ was cotreated with anticancer drugs, cisplatin, gemcitabine, methotrexate, paclitaxcel and vinblastine, that have been well studied in the patients with NF1-associated MPNSTs, in the HSC normal cells and S462 MPNST cells. After cotreatment of INF-γ and anticancer drugs for 48 h, cell viability was assessed (Figure 7). The results showed that co-treatment with INF-γ and cisplatin or INF-γ and gemcitabine significantly decreased cellular viability in S462 MPNST cells, but not in HSC cells.
Next, I further investigated a sensitization effect of INF-γ on combination treatment of cisplatin and gemcitabine. INF-γ was cotreated with cisplatin and gemcitabine for 48 h, cell viability was assessed (Figure 8). Notably, INF-γ produced a sensitization effect on enhancing cytotoxicity of MPNST cells by cotreatment of low-dose cisplatin and gemcitabine.
24
Figure 7. Effects of interferon-gamma on the sensitization of anticancer drugs in the MPNST cell lines. HSC cells (A) and S462 cells (B) were cotreated with 1,000 U/ml of interferon-γ (INF-γ) and following anticancer drugs for 48 h: 2 μg/ml of cisplatin (C), 5 μM of gemcitabine (G), 1 μM of methotrexate (M), 50 μM of paclitaxcel (P), or 100 mM of vinblastine (V). *p<0.05, **p<0.01, ***p<0.001
25
Figure 8. Sensitization effects of interferon-gamma on enhancing cytotoxicity of the MPNST cells cotreated with cisplatin and gemcitabine. HSC cells (A) and S462 cells (B) were cotreated with 1,000 U/ml of interferon-γ (INF-γ) and cisplatin (1 μg/ml) and gemcitabine (2.5 μM of gemcitabine) for 48 h. ***p<0.001
26
IV. Discussion
In this study, I found downregulation of the IFITM1 in the MPNST tissues from patients with NF1, primary-cultured MPNST cells, and MPNST cell lines (Figures 2 and 3). Interferon-induced transmembrane protein 1 (IFITM1 gene product) is also known as 9-27 or Leu13, and plays a key role in the antiproliferative effect of interferon-γ and cell adhesion signals (Akyerli et al., 2005; Yang et al., 2007).
It has been reported that transcription of IFITM1 is responsive to interferon-γ as well as interferon-α and its expression is down-regulated in low-grade diffuse astrocytomas and breast cancer (Hwang et al., 2003; Abba et al., 2004). The interferon-γ-induced decrease in the level of sarcoendoplasmic reticulum calcium
ATPase isoform 2 accounts for the depleted state of internal Ca2+ stores in
cytokine-treated human salivary gland cells (Meehan et al., 1997). Together with my data, these results suggest that interferon-γ related genes may play an important role in malignant progression of tumors. Indeed, several data have shown that interferon-γ treatment or transfection of interferon-γ gene directly inhibits the proliferation of MPNSTs or neurogibroma cell lines (Nakayama et al., 2003a; Nakayama et al., 2003b).
This study demonstrated that overexpression of IFITM1 by recombinant IFITM1 gene transfection and interferon-γ treatment caused a decrease in Ras activation and is downstream effector Erk1/2 (Figures 5, 6). These results indicate that the
27
IFITM1 level is closely involved in the Ras/Raf/Mek/Erk signaling pathway.
Neurofibromas and MPNSTs consist mostly of Schwann cells and fibroblasts, and they also contain other cell types, including perineural cells, mast cells, pericytes, endothelial cells, and smooth muscle cells (Gottfried et al., 2006).
Schwann cells are well known to contribute fundamentally to MPNST development (Carroll, 2012). Fibroblasts are associated with cancer cells in all stages of cancer progression through the production of growth factors, chemokines, and the extracellular matrix, and therefore fibroblasts are a key determinant in the malignant progression of cancer (Kalluri and Zeisberg, 2006). Current study
demonstrated that Schwann cells and fibroblasts (Figures 3, 4), indicating that two
of the major cells forming MPNST tumors, Schwann cells and fibroblasts, express
IFITM1. IHC results of IFITM1 in PN and MPNST tissues form patients with NF1
showed IFIT1 expressed in the cells expressing S100 (Table 1, Figure 3). Schwann
cells are characteristically express S100 protein (Weiss et al., 1983).IHC staining
using antibody against the SC lineage marker S100 showed that all tumors tested contained the S100 positive cells (Table 1) and more S100 positive cells were
existed in MPNSTs than PNs. Thus, the hypoexpression of IFITM1 in the
MPNSTS in this study may reflect the hypoexpression in these two types of cells.
The molecular mechanisms of IFITM1 in cancer development are still poorly understood. My data demonstrated that the inhibition of IFITM1 expression in normal-phenotypic primary cells and normal HSC cells by IFITM1 siRNA
28
treatment caused an increase in Ras and Erk1/2 activation, while the overexpression of IFITM1 in MPNST primary cells and cell lines by recombinant
IFITM1 construct transfection resulted in decreased Ras and Erk1/2 activation
(Figure 5). These results indicate that IFITM1 is closely involved in the
Ras-MAPK signaling pathway (Le and Parada, 2007; Rebecca and Smalley, 2014; Roof and Gutierrez-Hartmann, 2017).
All the data indicated that IFITM1 acts as tumor suppressor gene rather than as a proto-oncogene in NF1-associated MPNST development, which suggests that IFITM1 may be a good target molecule for therapeutic treatment and/or prevention of MPNSTs. Since reduced IFITM1 expression was accompanied by significantly increased RAS-MAPK signaling in MPNST cells, the augmentation of IFITM1 expression may attenuate MPNST development. Treatment of INF-γ in the MPNST cells caused elevated expression of IFITM1, thereby leading to a decrease in the Ras activation and its downstream Erk1/2 activation. Notably, INF-γ produced a sensitization effect on enhancing cytotoxicity of MPNST cells by cotreatment of low-dose cisplatin and gemcitabine. Collectively, INF-γ is a good candidate for elevating IFITM1 expression in MPNSTs, thereby leading to a decrease in the Ras-Erk pathway and subsequent producing a sensitization effect on anticancer drug treatment.
29
V. Conclusion
Interferon-induced transmembrane protein 1 (IFITM1) was downregulated in neurofibromatosis type 1 (NF1)-associated malignant peripheral nerve sheath tumor (MPNST) tissues compared to that in NF1-assocaited plexiform benign tissues. Downregulation of IFITM1 is closely involved in the activation of Ras/Raf/Mek/Erk signaling pathway that is essential for tumor progression in NF1.
Treatment of interferon-γ (INF-γ) in the MPNST cells caused elevated expression of IFITM1, thereby leading to a decrease in the Ras activation (GTP-Ras) and its downstream Erk1/2 activation (phosphorylated Erk1/2). Cotreatment of INF-γ and low-dose of cisplatin and gemcitabine effectively induced cytotoxicity of the MPNST cells.
30
References
1. Abba MC, Drake JA, Hawkins KA, Hu Y, Sun H, Notcovich C, Gaddis S, Sahin A, Baggerly K, Aldaz CM: Transcriptomic changes in human breast cancer progression as determined by serial analysis of gene expression. Breast
Cancer Res 6: R499-513, 2004
2. Akyerli CB, Beksac M, Holko M, Frevel M, Dalva K, Ozbek U, Soydan E, Ozcan M, Ozet G, Ilhan O, Gurman G, Akan H, Williams BR, Ozcelik T: Expression of IFITM1 in chronic myeloid leukemia patients. Leuk Res 29: 283-286, 2005
3. Borg D, Hedner C, Gaber A, Nodin B, Fristedt R, Jirstrom K, Eberhard J, Johnsson A: Expression of IFITM1 as a prognostic biomarker in resected gastric and esophageal adenocarcinoma. Biomark Res 4: 10, 2016
4. Boyd KP, Korf BR, Theos A: Neurofibromatosis type 1. J Am Acad Dermatol 61: 1-14; quiz 15-16, 2009
5. Brems H, Beert E, de Ravel T, Legius E: Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol 10: 508-515, 2009
6. Carroll SL: Molecular mechanisms promoting the pathogenesis of Schwann cell neoplasms. Acta Neuropathol 123: 321-348, 2012
7. Carroll SL, Ratner N: How does the Schwann cell lineage form tumors in NF1?
Glia 56: 1590-1605, 2008
8. Carroll SL, Stonecypher MS: Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors: II. The role of dysregulated growth factor signaling. J Neuropathol Exp Neurol 64: 1-9, 2005
31
M, Dunn D, Gesteland R, O'Connell P, et al.: A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell 62: 193-201, 1990
10. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A: Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med
Genet 39: 311-314, 2002
11. Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, Upadhyaya M, Towers R, Gleeson M, Steiger C, Kirby A: Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med
Genet 44: 81-88, 2007
12. Gottfried ON, Viskochil DH, Fults DW, Couldwell WT: Molecular, genetic, and cellular pathogenesis of neurofibromas and surgical implications.
Neurosurgery 58: 1-16; discussion 11-16, 2006
13. Gregorian C, Nakashima J, Dry SM, Nghiemphu PL, Smith KB, Ao Y, Dang J, Lawson G, Mellinghoff IK, Mischel PS, Phelps M, Parada LF, Liu X, Sofroniew MV, Eilber FC, Wu H: PTEN dosage is essential for neurofibroma development and malignant transformation. Proc Natl Acad Sci U S A 106: 19479-19484, 2009
14. Grobmyer SR, Reith JD, Shahlaee A, Bush CH, Hochwald SN: Malignant Peripheral Nerve Sheath Tumor: molecular pathogenesis and current management considerations. J Surg Oncol 97: 340-349, 2008
15. He J, Li J, Feng W, Chen L, Yang K: Prognostic significance of INF-induced transmembrane protein 1 in colorectal cancer. Int J Clin Exp Pathol 8: 16007-16013, 2015
16. Hwang IT, Kim YJ, Kim SH, Kwak CI, Gu YY, Chun JY: Annealing control primer system for improving specificity of PCR amplification. Biotechniques 35: 1180-1184, 2003
32
genes related to NF1-associated malignant transformation from a patient with neurofibromatosis. Genes & Genomics 30: 408-418, 2008
18. Jett K, Friedman JM: Clinical and genetic aspects of neurofibromatosis 1.
Genet Med 12: 1-11, 2010
19. Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K: The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl
Acad Sci U S A 102: 8573-8578, 2005
20. Jouhilahti EM, Peltonen S, Heape AM, Peltonen J: The pathoetiology of neurofibromatosis 1. Am J Pathol 178: 1932-1939, 2011
21. Kalluri R, Zeisberg M: Fibroblasts in cancer. Nat Rev Cancer 6: 392-401, 2006 22. Katz D, Lazar A, Lev D: Malignant peripheral nerve sheath tumour (MPNST): the clinical implications of cellular signalling pathways. Expert Rev Mol Med 11: e30, 2009
23. Kluwe L, Friedrich R, Mautner VF: Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes
Chromosomes Cancer 24: 283-285, 1999a
24. Kluwe L, Friedrich RE, Mautner VF: Allelic loss of the NF1 gene in NF1-associated plexiform neurofibromas. Cancer Genet Cytogenet 113: 65-69, 1999b
25. Le LQ, Parada LF: Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene 26: 4609-4616, 2007
26. Lee SJ, Park HJ, Kim YH, Kim BY, Jin HS, Kim HJ, Han JH, Yim H, Jeong SY: Inhibition of Bcl-xL by ABT-737 enhances chemotherapy sensitivity in neurofibromatosis type 1-associated malignant peripheral nerve sheath tumor cells. Int J Mol Med 30: 443-450, 2012
27. Legius E, Dierick H, Wu R, Hall BK, Marynen P, Cassiman JJ, Glover TW: TP53 mutations are frequent in malignant NF1 tumors. Genes Chromosomes
33
28. Levy P, Ripoche H, Laurendeau I, Lazar V, Ortonne N, Parfait B, Leroy K, Wechsler J, Salmon I, Wolkenstein P, Dessen P, Vidaud M, Vidaud D, Bieche I: Microarray-based identification of tenascin C and tenascin XB, genes possibly involved in tumorigenesis associated with neurofibromatosis type 1. Clin
Cancer Res 13: 398-407, 2007
29. Mawrin C, Kirches E, Boltze C, Dietzmann K, Roessner A, Schneider-Stock R: Immunohistochemical and molecular analysis of p53, RB, and PTEN in malignant peripheral nerve sheath tumors. Virchows Arch 440: 610-615, 2002 30. McCaughan JA, Holloway SM, Davidson R, Lam WW: Further evidence of
the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J Med Genet 44: 463-466, 2007
31. McClatchey AI: Neurofibromatosis. Annu Rev Pathol 2: 191-216, 2007
32. McQueen M, MacCollin M, Gusella J, Plotkin SR: Patient and physician attitudes regarding clinical trials in neurofibromatosis 1. J Neurosci Nurs 40: 341-345, 2008
33. Meehan S, Wu AJ, Kang EC, Sakai T, Ambudkar IS: Interferon-gamma induces a decrease in the intracellular calcium pump in a human salivary gland cell line. Am J Physiol 273: C2030-2036, 1997
34. Mo W, Chen J, Patel A, Zhang L, Chau V, Li Y, Cho W, Lim K, Xu J, Lazar AJ, Creighton CJ, Bolshakov S, McKay RM, Lev D, Le LQ, Parada LF: CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell 152: 1077-1090, 2013
35. Nakayama J, Tanaka T, Arakawa F, Terao H, Shimura H, Ikeda S, Kuroki M: Gamma interferon gene transfection efficiently inhibits proliferation of neurofibroma cell lines in vitro. J Dermatol 30: 181-188, 2003a
36. Nakayama J, Tanaka T, Furumura M, Takahashi A, Yamaguchi T, Shimura H, Ikeda S, Kuroki M: Inhibition of the proliferation of a malignant peripheral
34
nerve sheath tumor cell line by gamma interferon gene transfection. J
Dermatol 30: 879-885, 2003b
37. Nielsen GP, Stemmer-Rachamimov AO, Ino Y, Moller MB, Rosenberg AE, Louis DN: Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am J Pathol 155: 1879-1884, 1999
38. Park HJ, Lee SJ, Sohn YB, Jin HS, Han JH, Kim YB, Yim H, Jeong SY: NF1 deficiency causes Bcl-xL upregulation in Schwann cells derived from neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors.
Int J Oncol 42: 657-666, 2013
39. Rebecca VW, Smalley KS: Change or die: targeting adaptive signaling to kinase inhibition in cancer cells. Biochem Pharmacol 91: 417-425, 2014 40. Roof AK, Gutierrez-Hartmann A: Consider the context: Ras/ERK and
PI3K/AKT/mTOR signaling outcomes are pituitary cell type-specific. Mol Cell
Endocrinol, 2017
41. Rutkowski JL, Wu K, Gutmann DH, Boyer PJ, Legius E: Genetic and cellular defects contributing to benign tumor formation in neurofibromatosis type 1.
Hum Mol Genet 9: 1059-1066, 2000
42. Sari IN, Yang YG, Phi LT, Kim H, Baek MJ, Jeong D, Kwon HY: Interferon-induced transmembrane protein 1 (IFITM1) is required for the progression of colorectal cancer. Oncotarget 7: 86039-86050, 2016
43. Serra E, Rosenbaum T, Winner U, Aledo R, Ars E, Estivill X, Lenard HG, Lazaro C: Schwann cells harbor the somatic NF1 mutation in neurofibromas: evidence of two different Schwann cell subpopulations. Hum Mol Genet 9: 3055-3064, 2000
44. Spurlock G, Knight SJ, Thomas N, Kiehl TR, Guha A, Upadhyaya M: Molecular evolution of a neurofibroma to malignant peripheral nerve sheath tumor (MPNST) in an NF1 patient: correlation between histopathological,
35
clinical and molecular findings. J Cancer Res Clin Oncol 136: 1869-1880, 2010
45. Staser K, Yang FC, Clapp DW: Mast cells and the neurofibroma microenvironment. Blood 116: 157-164, 2010
46. Stricker TP, Henriksen KJ, Tonsgard JH, Montag AG, Krausz TN, Pytel P: Expression profiling of 519 kinase genes in matched malignant peripheral nerve sheath tumor/plexiform neurofibroma samples is discriminatory and identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed with transformation. Mod Pathol 26: 930-943, 2013
47. Thway K, Fisher C: Malignant peripheral nerve sheath tumor: pathology and genetics. Ann Diagn Pathol 18: 109-116, 2014
48. Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM: Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 65: 205-211, 2005
49. Upadhyaya M: Genetic basis of tumorigenesis in NF1 malignant peripheral nerve sheath tumors. Front Biosci 16: 937-951, 2011
50. Upadhyaya M, Kluwe L, Spurlock G, Monem B, Majounie E, Mantripragada K, Ruggieri M, Chuzhanova N, Evans DG, Ferner R, Thomas N, Guha A, Mautner V: Germline and somatic NF1 gene mutation spectrum in NF1-associated malignant peripheral nerve sheath tumors (MPNSTs). Hum Mutat 29: 74-82, 2008
51. Watanabe T, Oda Y, Tamiya S, Masuda K, Tsuneyoshi M: Malignant peripheral nerve sheath tumour arising within neurofibroma. An immunohistochemical analysis in the comparison between benign and malignant components. J Clin
Pathol 54: 631-636, 2001
52. Weiss SW, Langloss JM, Enzinger FM: Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest 49: 299-308, 1983
36
53. Yang G, Xu Y, Chen X, Hu G: IFITM1 plays an essential role in the antiproliferative action of interferon-gamma. Oncogene 26: 594-603, 2007 54. Yu J, Deshmukh H, Payton JE, Dunham C, Scheithauer BW, Tihan T, Prayson
RA, Guha A, Bridge JA, Ferner RE, Lindberg GM, Gutmann RJ, Emnett RJ, Salavaggione L, Gutmann DH, Nagarajan R, Watson MA, Perry A: Array-based comparative genomic hybridization identifies CDK4 and FOXM1 alterations as independent predictors of survival in malignant peripheral nerve sheath tumor. Clin Cancer Res 17: 1924-1934, 2011
55. Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF: Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science 296: 920-922, 2002
37 -국문 요약- 제1형 신경섬유종증의 종양 악성화에 관여하는 IFITM1 유전자의 역할 아주대학교 대학원 의생명과학과 임 규 빈 (지도교수: 김 현 주, 정 선 용) 제1형 신경섬유종증 (Neurofibromatosis type 1, NF1) 은 3,000~3,500명당 1명 꼴로 발생하는 가장 흔한 유전질환 중 하나이다. 양성종양인 neurofibroma가 악성종양인 malignant peripheral nerve sheath tumor(MPNST) 로의 발전은 전체 환자의 10~13%에 이르고, MPNST는 NF1 환자의 주요 사망원인으로 보고되고 있다. 하지만 neurofibroma의 MPNST로의 악성화 기전은 아직 명확하게 규명되어 있지 않다. 선행연구에서 NF1 환자의 수술 조직 유래의 정상, 양성종양, 악성종양 조직에서 발현의 차이가 큰 유전자로서 IFITM1(interferon-induced transmembrane protein 1)이 발굴되었다. 본 연구에서는 NF1의 종양 악성화 에 관여하는 IFITM1의 역할에 대해 규명하고자 하였다 먼저 7명의 NF1 환자의 수술조직에서 양성종양조직(n=6)과 악성종양조 직(n=3)에서 IFITM1 발현 정도를 면역학적 염색법(immunohistochemistry) 을 통해 조사하였다. 그 결과 양성종양조직에 비하여 악성종양조직에서 IFITM1 단백질의 양이 확연히 적은 것을 확인하였다. 특히 2명의 환자에 서는 시간 경과에 따라 IFITM1이 감소되는 것을 확인 하였다. 다음으로, 양성종양에서 악성종양으로 발전한 환자 수술조직으로부터 분리 배양한
38
정상세포와 악성종양의 MPNST 세포에서 IFITM1 mRNA와 단백질 양을 비교·분석한결과 IHC 결과와 동일하게 악성종양세포에 IFITM1의 발현 량이 감소되어 있는 것을 확인 하였다.
IFITM1의 발현량이 NF1의 악성화에 직접적인 영향이 있는지 증명하기 위해, siRNA 처리를 통한 IFITM1의 발현 억제와 plasmid construct 도입을 통한 IFITM1의 과발현(overexpression)에 대한 연구를 진행하였다. 환자유 래의 정상세포와 정상의 human Schwann cell(HSC)에 IFITM1 발현을 억제 하였을 때 Ras 신호전달의 하류에 있는 Erk-1/2의 활성화가 확인 되었으 며, 반대로 환자유래의 악성세포와 MPNST 세포주에 IFITM1을 과발현시 켰을 때 Ras 활성화(GTP-Ras)가 현저히 증가되었다. 이러한 결과는 IFITM1의 발현량이 NF1의 악성화와 밀접한 영향이 있음을 시사한다.
IFITM1은 인터페론-알파(interferon-α)와 인터페론-감마(interferon-γ)에 의
해 전사가 유도되는 유전자로 알려져 있다. 이에 본연구에서도 MPNST 세포주에 인터페론-감마를 처리하여 IFITM1의 발현량 변화와 이에 따른 Ras 및 Erk 1/2의 활성 변화를 조사하였다. sNF96.2 MPNST 세포에 인터페 론-감마를 처리한 3시간 후에 관찰하였을 때, 현저한 IFITM1의 발현의 증가가 관찰되었으며 활성화된 Ras(GTP-Ras)와 활성화된 Erk 1/2 (phosphorylated Erk 1/2)의 현저한 감소가 있음이 밝혀졌다. MPNST 환자에서 많이 사용되고 있는 항암제인, cisplatin, gemcitabine, methotrexate, paclitaxcel, vinblastine을 단독 처리와 인터페론-감마 병용처리 에 대한 비교 실험에서, 인터페론-감마는 cisplatin과 gemcitabine에 대한 감작(sensitization)에 큰 영향을 끼치는 것으로 나타났다. 특히, cisplatin과 gemcitabine의 병용 처리시에 인터페론-감마의 cisplatin과 gemcitabine에 대 한 감작 효과가 가장 좋았다.
39 인터페론-감마에 의해 유도된 IFITM1의 발현량 증가는 MPNST 세포의 Ras/Raf/Mek/Erk 경로의 활성화 억제에 영향을 미치는 것을 알 수 있다. IFITM1에 관한 본 연구의 결과가 향후 NF1 악성화 원인규명 및 치료제 개발에 기여할 수 있기를 기대한다. --- 핵심어: 신경섬유종증 제1형 (NF1), 악성말초신경초종양(MPNST), 종양진행, IFITM1, Ras, Erk1/2, 인터페론-감마