의
의
의
의학
학
학 석
학
석
석
석사
사
사
사학
학위
학
학
위
위
위 논
논
논문
논
문
문
문
Role of the Nfa1 Protein in
Pathogenic Naegleria fowleri
Co-cultured with Target Cells
아
아
아
아 주
주
주 대
주
대
대
대 학
학
학 교
학
교 대
교
교
대
대
대 학
학
학 원
학
원
원
원
의
의
의
의 학
학
학 과
학
과
과
과
강
강
강
강 수
수
수 연
수
연
연
연
강
강
강
강수
수연
수
수
연
연
연의
의
의
의 의
의학
의
의
학
학
학 석
석
석사
석
사
사학
사
학위
학
학
위
위
위 논
논
논
논문
문
문
문을
을 인
을
을
인
인
인준
준
준
준함
함
함
함....
심
심
심
심사
사
사위
사
위
위
위원
원
원
원장
장
장
장
신 호
신
신
신
호
호
호 준
준
준
준
인
인
인
인
심
심
심
심 사
사
사
사 위
위
위
위 원
원
원
원
이 재
이
이
이
재
재
재 호
호
호
호
인
인
인
인
심
심
심
심 사
사
사
사 위
위
위 원
위
원
원
원
박
박
박
박
선
선
선
선
인
인
인
인
아
아
아
아 주
주
주 대
주
대
대
대 학
학
학 교
학
교 대
교
교
대
대
대 학
학
학 원
학
원
원
원
2222000000004444년
년
년
년 11112222월
월
월 22222222일
월
일
일
일
Role of the Nfa1 Protein in
Pathogenic Naegleria fowleri
Co-cultured with Target Cells
by
Su-Yeon Kang
A Dissertation Submitted to The Graduate School of Ajou University
In Partial Fulfillment of the Requirements for the Degree of
MASTER OF MEDICAL SCIENCES
Supervised by
Ho-Joon Shin, Ph.D.
Department of Medical Sciences
The Graduate School, Ajou University
i
- ABSTRACT -
Role of the Nfa1 Protein in Pathogenic Naegleria fowleri
Co-cultured with Target Cells
Naegleria fowleri, a free-living amoeba, exists as a virulent pathogen which
causes fatal primary amoebic meningoencephalitis (PAME) in experimental animal and humans. Using infected and immune mouse sera, we previously cloned an antigenic gene (called nfa1) from a cDNA library of N. fowleri by immunoscreening. The nfa1 gene had the coding sequence of 360 bp, producing a 13.1kDa recombinant protein (rNfa1). Anti-Nfa1 polyclonal antibody, revealed pseudopodia-specific immunolocalization of Nfa1 protein in a trophozoite of N. fowleri. An anti-Nfa1 antibody showed a neutralizing effect on the cytotoxicity of N. fowleri trophozoites against CHO cells, much like treating an anti-Nfa1 antibody on a cocultivating system. In spite of the wide use of N. fowleri in free-living amoebic pathogenicity, no informations what proteins are involved in the functions of these organisms are yet available. In this study, we observed the role of Nfa1 protein in a cell-contact mechanism of pathogenic N. fowleri cocultured with target cells (CHO cell) by the immunofluorescence assay. Using confocal microscopic findings, the Nfa1 protein located on pseudopodia of N. fowleri trophozoites. The Nfa1 protein in N. fowleri trophozoites co-cultured with CHO cells was located on pseudopodia and in a food-cup formed as a phagocytic structure close contact with target cells. The amount of
nfa1 mRNA of N. fowleri was strongly increased at 6 hr post co-culture. Finally, it
was elucidated that the Nfa1 protein played an important role in phagocytic activity, a cell-contact mechanism of pathogenic N. fowleri.
Key words : Naegleria fowleri, nfa1 gene, immunolocalization, Nfa1 protein
iii
TABLE OF CONTENTS
ABSTRACT ………... i
TABLE OF CONTENTS ………... iii
LIST OF FIGURES ………... v
ABBREVIATIONS ………... vii
I. INTRODUCTION ………... 1
II. MATERIALS AND METHODS ……….... 5
A. Cultivation of N. fowleri and CHO cells ……….... 5
B. Expression of the nfa1 gene and production of a rNfa1 protein ……… 5
C. Production of an anti-Nfa1 polyclonal antibody ……….... 6
D. Immunofluorescence and confocal microscopy ………... 7
E. Northern blot analysis ... 10
III. RESULTS ……… 11
A. Production of a recombinant Nfa1 fusion protein ………...…... 11
B. Characterization of an anti-Nfa1 polyclonal antibody ………. 12
C. Immunostaining of N. fowleri trophozoites and CHO cells with an anti-Nfa1 polyclonal antibody ……… 14
D. Counter-staining of N. fowleri trophozoites and CHO cells to observe the localization of the Nfa1 protein ..………... 16
E. Localization of the Nfa1 protein of N. fowleri trophozoites co-cultured with CHO cells ……… 19
F. Profile of the nfa1 mRNA of N. fowleri in co-culture system ...23 IV. DISCUSSION ………. 24 V. CONCLUSION ……… 27 BIBLIOGRAPHY ………. 28 국문요약 ……….. 32
v
LIST OF FIGURES
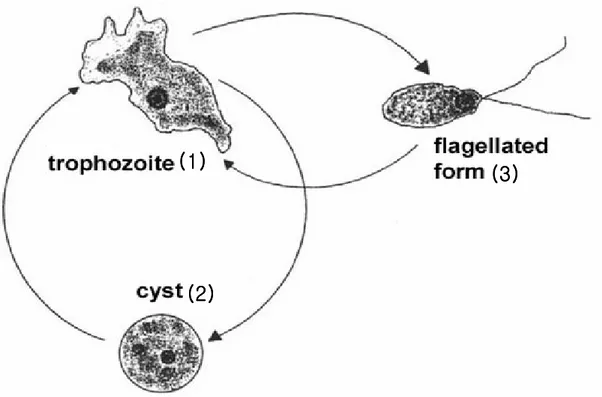
Fig. 1. The life cycle of Naegleria fowleri ... 2
Fig. 2. Naegleria fowleri trophozoites stained only with the fluorescein
isothiocyanate(FITC)-conjugated anti-mouse immunoglobulin G…... 8
Fig. 3. CHO cells stained with the fluorescein isothiocyanate(FITC)-
conjugated anti- mouse antibody ………. 9
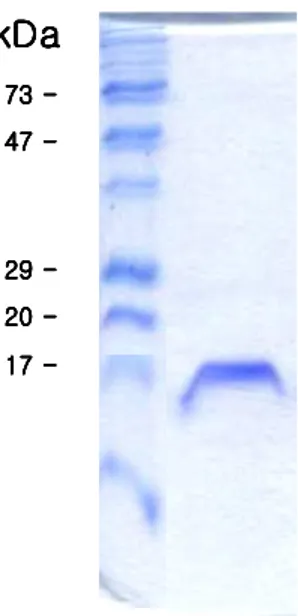
Fig. 4. SDS-PAGE band pattern of a recombinant Nfa1 fusion protein
expressed from an nfa1 gene ………... 11
Fig. 5. Western blotting band patterns of a recombinant Nfa1 protein with
an anti-Nfa1 polyclonal antibody and healthy mouse serum... 13
Fig. 6. Confocal microscopic findings of Naegleria fowleri trophozoites only
and co-cultured with CHO cells ...………..……….. 15
Fig. 7. Counter-staining of Naegleria fowleri trophozoites to observe the
Fig. 8. CHO cells stained with 5-(and-6)-chloromethyl
SNARF-1 (red) ... 18
Fig. 9. Confocal microscopic findings of Naegleria fowleri trophozoites
co-cultured with CHO cells for 1 hr………... 20
Fig. 10. Confocal microscopic findings of Naegleria fowleri trophozoites
co-cultured with CHO cells for 3 hr ………..………...…... 21
Fig. 11. Confocal microscopic findings of Naegleria fowleri trophozoites
co-cultured with CHO cells for 6 hr …..……….……….……….. 22
Fig. 12. Northern blot findings by an anti-Nfa1 antibody of sRNA extracted from Naegleria fowleri trophozoites and/or
vii
ABBREVIATIONS
CHO Chinese hamster ovary CNS Central nervous system DMSO Di methyl sulfoxide
EMEM Earle’s minimum essential medium FBS Fetal bovine serum
FITC Fluorescein isothiocyanate IFA Immunofluorescence assay IPTG Isopropylthiogalactoside
PAME Primary amoebic meningoencephalitis PBS Phosphate buffered saline
І. INTRODUCTION
Naegleria, a free-living amoeba, is commonly found in soil and in warm bodies
of fresh water, such as lakes, rivers and hot springs, unchlorinated swimming pools, and in warm water discharge pools from industrial plants (Culbertson, 1971 and Kollars et al., 1996). Only one species of them N. fowleri, has been found to infect humans.
N. fowleri has three stages, cyst, trophozoites, and flagellated forms, in its life
cycle (John, 1982) (Fig. 1). N. fowleri trophozoites ranges 7-20 µm in size. They have a single nucleus with a large karyosome and no peripheral chromatin. The cysts have a single nucleus that is almost identical to that seen in the trophozoite (Visvesvara et al., 1999).
N. fowleri produces an acute lethal central nervous system (CNS) disease called
primary amoebic meningoencephalitis (PAME). As of 1990, approximately 200 cases of amoebic meningoencephalitis had been reported world-wide. Of these, approximately 75% have been PAME and 25% due to acanthamoebic encephalitis (Seidel et al., 1982; Im et al., 2003). Fewer than 100 cases of PAME have been reported in the United States in the 25 years since these diseases were recognized. PAME lead to death in most cases (Seidel et al., 1982; John, 1982). The mortality of patients with PAME is greater than 95% because of the rapid progression of the disease, delayed diagnosis, and the lack of effective therapeutic agents (Barnett et al., 1996).
- 2 -
Fig. 1. The life cycle of Naegleria fowleri. N. fowleri changes its shape according to the environmental changes. Trophozoites stages (1) and cyst stage (2) are formed in normal condition and under conditions of nutritional depletion, respectively. Flagellate stage (3) is formed transiently in specific-media condition.
(1)
(1)
(1)
(1)
(2)
(2)
(2)
(2)
(3)
(3)
(3)
(3)
(1)
(1)
(1)
(1)
(2)
(2)
(2)
(2)
(3)
(3)
(3)
(3)
N. fowleri trophozoites penetrate the nasal mucosa and enter the brain through the
cribriform plate. The organisms are capable of multiplying in the tissues of the CNS and may be isolated from spinal fluid. In untreated cases, death occurs within 1 week of the onset of symptoms. Because their pathogenic lesions are mainly in the CNS and PAME progresses rapidly, it is difficult to diagnose PAME in its early stages (Ma et al., 1990). For the diagnosis, the antigen-related gene producing the antigenic molecule has been largely unsuccessful.
To obtain antigenic molecules for use as diagnostic agents in pathogenic N.
fowleri infection, an antigenic gene (called nfa1) cloned from a cDNA library of N. fowleri by immunoscreening using infected and immune mouse sera (Shin et al.,
2001). The nfa1 gene had a coding nucleotide sequence of 360 bp, producing a recombinant protein (rNfa1) of 13.1 kDa (Shin et al., 2001). An anti-Nfa1 polyclonal antibody obtained from mice immunized with an rNfa1 protein was used in immunocytochemistry, showing the Nfa1 protein as an indicator of the pseudopodia-specific immunolocalization on a trophozoite of N. fowleri (Cho et al., 2003). An anti-Nfa1 antibody causes a decreasing effect on the cytotoxicity of N. fowleri trophozoites against CHO (Chinese hamster ovary) cells and the proliferation of N.
fowleri trophozoites in a dose-dependent manner (Jeong et al., 2004), much like
treating an anti-Nfa1 antibody on a co-cultivating system (Cho et al., 2003).
In the process of host-tissue invasion, the adherence of amoeba to host cells is the most important step in the mechanism of pathogenicity of N. fowleri. A specific pseudopodial projection, called a amoebastome, is formed (Marciano-Carbral, 1988).
- 4 -
In spite of the widespread use of free-living amoebae understand protozoan pathogenicity, no informations is yet fully available regarding the presence of pathogen-related proteins and their functions in these organisms. Moreover, the role of a newly cloned Nfa1 protein has not been carried out.
In this study, by observing the localization of the Nfa1 protein in the co-culture system with target cells by the immunofluorescence assay, we observed the role of the Nfa1 protein in a cell-contact mechanism of N. fowleri. In addition, the transcription of nfa1 mRNA of N. fowleri during the co-culture system was identified by Northern blot with an nfa1-specific probe.
ІІ. MATERIALS AND METHODS
A. Cultivation of N. fowleri and CHO cells
N. fowleri trophozoites (Cater NF69 strain, ATCC NO.30215) were axenically
cultured in Nelson’ media at 37 °C (Willaert, 1971). CHO cells were cultured with EMEM (Earle’s Minimum Essential Medium) containing 10% fetal bovine serum (complete EMEM) at 37°C in a 5% CO2incubator, in accordance with the methods
of a previous paper (Jeong et al., 2004).
B. Expression of a nfa1 gene and production of an rNfa1 protein
To obtain an rNfa1 fusion protein, the expression of a nfa1 gene and the purification of a recombinant protein were performed accordingly by the method mentioned in a previous paper (Shin et al., 2001). The purified DNA (5 µg/µl) obtained from PCR-T7/NT TOPO expression vector (Invitrogen, Grohingen, Netherlands) containing a nfa1 gene was subsequently transformed into the BL21(DE3) pLysS E. coli strain by the heat-shock method. Cells were cultured at 37°C in the LAC (Luria-Bertani media containing 100 g/ml of ampicillin and 34 g/ml of chloramphenichol) plates for selection. A transformed-colony was selected and cultured in the LAC broth at 37°C until the absorbance reached 0.5-0.8 at 600 nm, 1 mM IPTG was added to the media. After 4 hr of incubation, the cells were
- 6 -
harvested by centrifugation (6,000 × g for 15 min). Cell extracts were compared with those of non-transformed BL21(DE3) pLysS by SDS-PAGE, and the presence of the expressed gene product was confirmed by Western blot using both the immune and the infected sera, and anti-His and Xpress antibodies (Invitrogen).
C. Production of an anti-Nfa1 polyclonal antibody
For the production of anti-Nfa1 polyclonal serum, the rNfa1 protein (50
µg/mouse) was mixed with an equal volume of complete Freund’s adjuvant (Sigma) and injected intraperitoneally into an 8-week-old female BALB/c mice (purchased from the Korea Institute of Science and Technology, Daejeon, Korea). The mice were boosted biweekly for another 4 weeks with the rNfa1 protein (25 µg/mouse) containing an equal volume of incomplete Freund’s adjuvant (Sigma). After the third boosting, the rNfa1 protein (5 µg/mouse) was injected intravenously without the adjuvant. Four days later, an anti-Nfa1 polyclonal serum was collected from the blood by centrifugation at 2,500 × g for 30 min at 4°C. ELISA was performed with a purified Nfa1 protein (5 µg/ml) and with a rabbit anti-mouse whole immunoglobulin (1:10,000 dilution) conjugated with alkaline phosphate (Sigma). Western blotting was performed for the rNfa1 protein, in accordance with the method described in a previous paper (Jeong et al., 2004).
D. Immunofluorescent assay and confocal microscopic practice
For immunofluorescence studies, cultivating trophozoites of N. fowleri or CHO cells used as target cells were fixed in 10% formaldehyde for 30 min at room temperature, permeabilized in 1% ammonium hydroxide, washed in Tween 20 for 5 min and extensively washed with 0.82% saline. N. fowleri was incubated overnight at 4 °C with an anti-Nfa1 polyclonal antibody diluted serially with PBS (pH 7.4). This was followed by incubation with the appropriate fluorescein isothiocyanate (FITC)- conjugated anti-mouse antibody (Sigma. USA) at a dilution of 1:100 for 2 hr at 4 °C. As the controls, N. fowleri trophozites and CHO cells stained with the fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibodies (Fig. 2,3). For labeling the living target cells, CHO cells were incubated 1 hr at 37 °C. DMSO stock solutions (100 µl in SNARF stock) are typically diluted 1:1,000 into loading buffer (20 µl in PBS) to reduce the exposure of cells to DMSO. The loading buffer should be serum-free because serum often contains esterase activity. Total fluorescence of cells was visualized in optical section produced by Olympus FV-500 confocal microscope (Olympus). The collected images were processed using Adobe Photoshop 7.0 software.
- 8 -
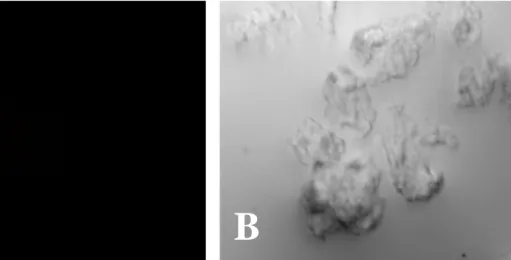
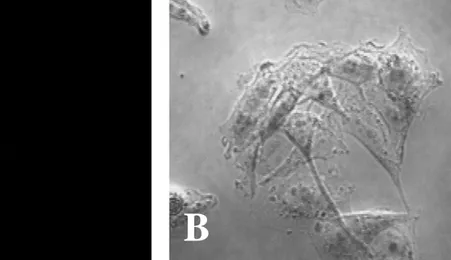
Fig. 2. Naegleria fowleri trophozoites stained only with the fluorescein isothiocyanate(FITC)-conjugated anti-mouse immunoglobulin G. No fluorescence of N. fowleri trophozoites was seen by a fluorescent microscope (A). B, light microscopic finding of N. fowleri trophozoites. (x 400).
A
B
A
Fig. 3. CHO cells stained with the fluorescein isothiocyanate(FITC)-conjugated anti- mouse antibody. No fluorescence of CHO cells was seen by a fluorescent microscope (A). B, light microscopic finding of CHO cells. (x 400).
A
B
A
- 10 -
E. Northern blot analysis
In order to observe the change of an nfa1 mRNA transcription in N. fowleri cocultured with target cells, total RNA of N. fowleri trophozoites and CHO cells was extracted using the RNAzol B reagent (TEL-TEST, Firndswood, Tx, USA) and used for Northern blot analysis. Briefly, each cell (2 × 105
) was collected in a sterile 15 ml conical tube, centrifuged at 300 g for 5 min and washed once with PBS. The pellet was lysed with 500 µl of RNAzol B reagent, and RNA was extracted with 50 µl of chloroform. The tube was inverted 10 times and cooled on ice for 5 min. After centrifugation at 10,000 g at 4 °C for 20 min, aqueous phase, which contained RNA, was transferred to a fresh tube and precipitated with 250 µl of isopropanol at 4 °C for 2 hr. After centrifugation at 10,000 g at 4 °C for 8 min, RNA pellets were washed in 80% ethanol, centrifuged, dried by speed-vacutainer, and finally dissolved in 50 µl of DEPC-treated DW. In order to perform the Northern blot technique, total RNA (30 µg) was denatured and electrophoresed on a 1% agarose gel containing formaldehyde, and blotted onto a nylon membrane. RNA on the membrane was hybridized with a 32P-labeled random-primed probe specific for the nfa1 gene.
III. RESULTS
A. Production of a recombinant Nfa1 fusion protein
For the expression of a nfa1 gene using a E. coli expression system, a nfa1 was inserted PCR-T7/NT TOPO vector. It was introduced into the E. coli strain BL21 and expressed by IPTG induction. The 17-kDa fusion protein was purified from E. coli extracts and confirmed by SDS-PAGE (Fig. 4).
Fig. 4. SDS-PAGE band pattern of a recombinant Nfa1 fusion protein expressed from an nfa1 gene.
kDa kDa kDa kDa 73 73 73 73 --- -47 47 47 47 --- -29 29 29 29 --- -20 20 20 20 --- -17 17 17 17 --- -kDa kDa kDa kDa 73 73 73 73 --- -47 47 47 47 --- -29 29 29 29 --- -20 20 20 20 --- -17 17 17 17 ---
-- 12 --
B. Characterization of an anti-Nfa1 polyclonal antibody
To obtain a purified recombinant Nfa1 protein, a 17 kDa fusion protein was purified using a nickel resin column and enzyme digestion. Finally, the purified protein eluted from the gels had a of 13.1 kDa, which corresponded well to the size in the previous research (Fig. 5). Anti-Nfa1 polyclonal antibodies were collected from mice immunized with rNfa1 protein. The specific reactivity against the Nfa1 protein was observed with ELISA and Western blotting (Fig. 5). Values of anti-Nfa1 polyclonal antibodies by ELISA ranged from 0.212 ± 0.021 (1:10,000 dilution, M ± SD, N = 5) to 1.213 ± 0.024 (1:200 dilution), which showed a difference from the 0.112 ± 0.005 (1:200 dilution) of the normal sera and also the 0.109 ± 0.005 of the PBS control.
Fig. 5. Western blotting band patterns of a recombinant Nfa1 protein with an anti-Nfa1 polyclonal antibody (A) and healthy mouse serum (N). Arrow, uncut 17 kDa protein; Arrow head, cut 13.1 kDa protein; M, Molecular weight marker
A
N
- 14 -
C. Immunostaining of N. fowleri trophozoites and CHO cells with an anti-Nfa1 polyclonal antibody
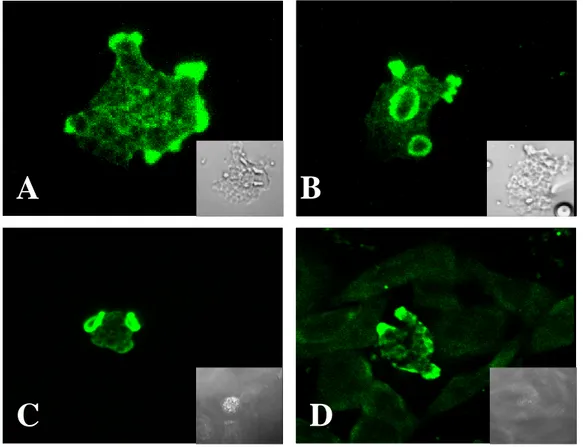
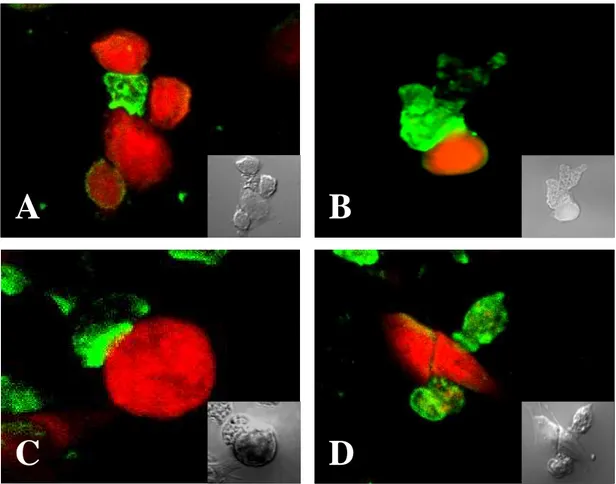
The presence of Nfa1 protein in amoebas fixed during locomotion was initially examined by staining amebae with an anti-Nfa1 polyclonal antibodies and FITC-conjugates, and by recording their total fluorescence in a Confocal microscope. By confocal microscopic findings, the Nfa1 protein of N. fowleri trophozoites in cultivation on Nelson’s medium was concentrated in the pseudopodia (Fig. 6A) and protrusive food-cup (Fig. 6B). Subsequently, in N. fowleri co-cultured with CHO cells, the Nfa1 protein was shown in food-cups, as well as in the pseudopods of amoeba (Fig. 6C, D). In contrast, no fluorescent signals were seen in CHO cells (Fig. 6C, D).
Fig. 6. Confocal microscopic findings of Naegleria fowleri trophozoites only and co-cultured with CHO cells. Strong fluorescent signals were shown in pseudopodia, especially food-cups, of N. fowleri trophozoites in media (A, B) and co-cultured with CHO cells (C, D). The light microscopic findings were showed in each box. (x 400).
A
B
A
C
D
A
B
A
B
A
C
D
- 16 -
D. Counter-staining of N. fowleri trophozoites and CHO cells to observe the localization of the Nfa1 protein
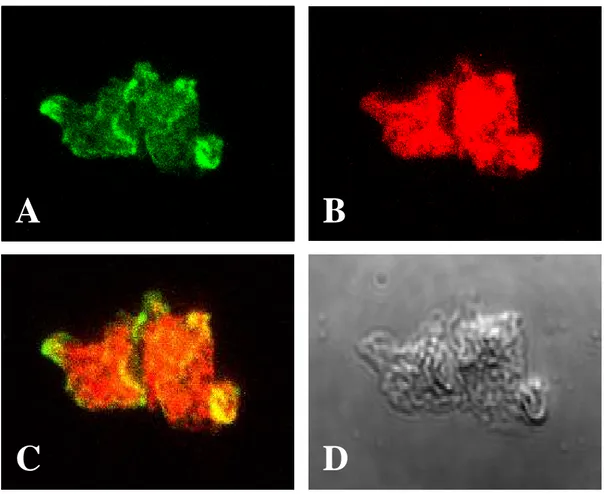
In this experiments, to analyse the localization and function of the Nfa1 protein, and to verify that an anti-Nfa1 antibody recognize a Nfa1 proteins in the pseudopodia and food-cup, the competitive counter-staining for amoeba and CHO cells was performed. In the counter-staining of N. fowleri with an anti-Nfa1 antibody using FITC-conjugate (Fig. 7A) and with the 5-(and-6)-chloromethyl SNARF-1 which was used for staining of CHO cells (Fig. 7B), strong signal of the Nfa1 proteins showed apparently in amoebic pseudopodia and food-cup. These results clearly indicate that there are no competition between an anti-Nfa1 polyclonal antibody and 5-(and-6)-chloromethyl SNARF-1 used for staining CHO cells. In addition, to identify the Nfa1 protein in N. fowleri trophozoites co-cultured with CHO cells and to detect its localization during cell contact, target cell (CHO cells) were counter stained with 5-(and-6)-chloromethyl SNARF-1 showed red color (Fig. 8).
Fig. 7. Counter-staining of N. fowleri trophozoites to observe the localization of the Nfa1 protein. N. fowleri trophozoite was reacted with an anti-Nfa1 antibody and stained with FITC (green) (A) and SNARF-1 (red) (B). The Nfa1 protein showed on pseudopodia and food-cups by a fluorescent microscope. C, their colocalization in the merged picture. (yellow); D, A light microscopic findings. (x400).
A
B
C
D
A
A
B
B
C
C
D
D
- 18 -
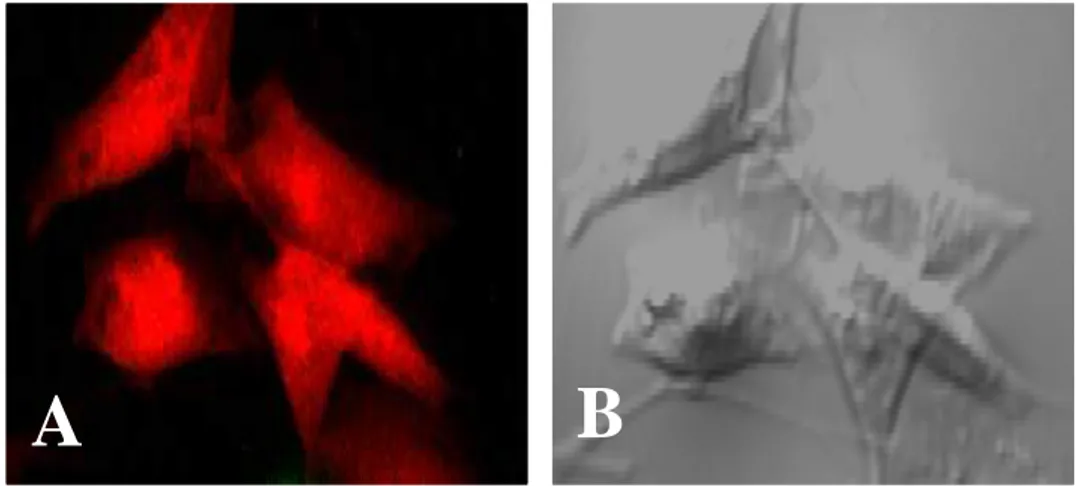
Fig. 8. CHO cells stained with 5-(and-6)-chloromethyl SNARF-1 (red). A, Red color from CHO cells observed by a fluorescent microscope;
B, light microscopic finding. (x 400).
A
B
A
E. Localization of the Nfa1 protein of N. fowleri trophozoites co-cultured with CHO cells
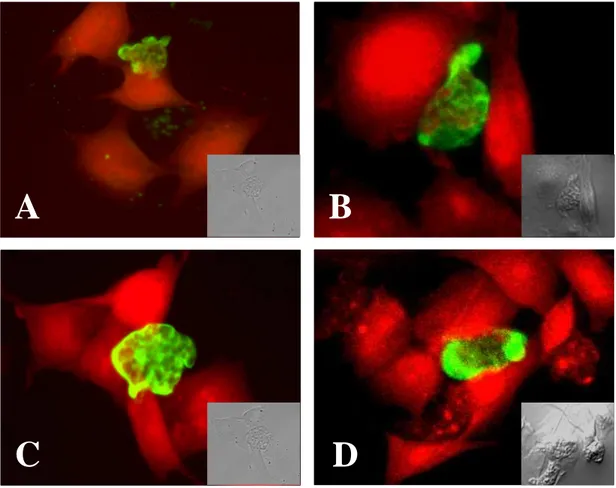
To observe the role of the Nfa1 protein of N. fowleri trophozoites co-cultured with target cells, N. fowleri and CHO cells were co-cultured at the time course of 1hr, 3hr, and 6hr. After 1 hr of cultivation, the Nfa1 protein of N. fowleri trophozoites concentrated at the point of contact with CHO cells. Strong fluorescent signals were shown especially in pseudopodia of N. fowleri trophozoites (Fig. 9). The food-cups of N. fowleri trophozoites has been found. Instead, the Nfa1 protein often were observed on the area in contact with the target cells (Fig. 9). After 3 hr of cultivation,
N. fowleri trophozoites showed continuing attachment on the surface of CHO cells
(target cells). Strong fluorescent signals were shown in pseudopodia (Fig. 10A, B, C) and food-cup (Fig. 10D) of N. fowleri trophozoites. At 6 hr of cultivation, the fluorescence around the pseudopodia contacted with target cells was distinguishable from the background fluorescence. Also, strong fluorescent signals were shown in pseudopodia, especially food-cups of N. fowleri trophozoites (Fig. 11). Several red-colored spots that seemed to fragmented CHO cell debris are shown in N. fowleri trophozoites (Fig. 11C).
- 20 -
Fig. 9. Confocal microscopic findings of Naegleria fowleri trophozoites co- cultured with CHO cells for 1 hr. Strong fluorescent signals were shown in
pseudopodia of trophozoites of N. fowleri (A, B, C, D). CHO cells were stained with SNARF-1. The light microscopic findings were shown in each box. (x 400).
A
B
C
D
A
A
B
B
C
C
D
D
Fig. 10. Confocal microscopic findings of Naegleria fowleri trophozoites co- cultured with CHO cells for 3 hr. Strong fluorescent signals were shown in pseudopodia (A, B, C) and food-cup (D) of trophozoites of N. fowleri. CHO cells were stained with SNARF-1. The light microscopic findings were shown in each box. (x 400).
A
B
C
D
A
A
B
B
C
C
D
D
- 22 -
Fig. 11. Confocal microscopic findings of Naegleria fowleri trophozoites co- cultured with CHO cells for 6 hr. Strong fluorescent signals were shown in pseudopodia and food-cups (A, B, C) of trophozoites of N. fowleri. CHO cells were stained with SNARF-1. The light microscopic findings were shown in each box (x400).
A
B
C
D
A
A
B
B
C
C
D
D
F. Profile of the nfa1 mRNA of N. fowleri in co-culture system.
To observe the transcription of the nfa1 mRNA, total RNA was extracted from N.
fowleri trophozoites and CHO cells in co-culture system (Fig. 12A). According to
Northern blot using an nfa1 gene-specific probe, the nfa1 mRNA was transcribed better in N. fowleri in the co-culture system than was N. fowleri only. Moreover, the
nfa1 mRNA was not transcribed in CHO cells. In particular, the amount of nfa1
mRNA of N. fowleri was increased strongly at 6 hr post co-culture (Fig. 12B).
Fig. 12. Northern blot findings by an anti-Nfa1 antibody of sRNA extracted from Naegleria fowleri trophozoites and/or CHO cells. A, Photographic findings of 18s RNAs extracted from Naegleria fowleri trophozoites and/or CHO cells. B, The nfa1 mRNA was strongly transcribed at 6 hr post coculture. Lane 1, CHO cells only; Lane 2, N. fowleri cultured with CHO cells for 3 hr; Lane 3, N. fowleri co-cultured with CHO cells for 6 hr; Lane 4, N. fowleri co-co-cultured with CHO cells for 12 hr; Lane 5, N. fowleri only.
A
B
C H O 3 hr 6 hr N f C o -cu ltureA
B
C H O 3 hr 6 hr N f C o -cu lture- 24 -
IV. DISCUSSION
N. fowleri is a free-living amoeboflagellate of soiland freshwater and exists all over the world. Although amoebic trophozoites are able to fulfill their life cycle without the interventionof a parasitic stage, they are invade the human brain via the nasal mucosa and olfactory nerve, and cause PAME (Marciano-Cabral, 1988; Shin et al., 2004). Consequently, N. fowleri is cytopathogenic to a variety of cultured mammalian cells (Willaert, 1971; Marciano-Cabral, 1988; Shin et al., 2004).The proposed mechanism(s) of the cytopathic action of this organism for mammalian cells includes active phagocytosis of cells by pseudopodia formation (Ma et al., 1990).Concerned with host-tissue invasion, the adherence of the amoeba to host cells is the most important step in mechanism of N. fowleri, a specific pseudopodial projection, called an amoebastome, is formed (Marciano-Cabral, 1988).
In the previous study, the immunolocalization study of the Nfa1 protein revealed that this protein was abundant in pseudopodia and around food vacuoles of N. fowleri trophozoites (Cho et al., 2003).This suggests that the Nfa1 protein is required for amoeba movement and food ingestion. It provides no information about N. fowleri and co-cultured with target cells that are specialized to different functions which comprise the mechanism of N. fowleri pathogenic activities: cell surface contraction, frontal pseudopodia polymerization and protrusive activity, cell anchoring to the substratum.
In this experiment, it was shown that the Nfa1 protein as a cell-contact mechanism in N. fowleri co-cultured with target cells was involved. By confocal microscopic findings, the Nfa1 protein of N. fowleri trophozoites co-cultured with CHO cells was located on pseudopodia in contact with target cells and in a food-cup formed as a phagocytic structure. In addition, the amount of nfa1 mRNA of N.
fowleri was increased at 6 hr post co-culture by Northern blotting with an
nfa1-specific probe. In other words, it seemed that the Nfa1 protein played a role in the
formation as a phagocytic activity. Pathogenicity is the ability of a micro-organism to produce disease, whereas
cytopathogenicity is the ability to produce pathologic change in cells or a cytopathic effect in vitro in cultured cells. The proposed mechanisms of cytopathogenicity for N.
fowleri include phagocytosis, release of cytolytic substances and the presence of a
biologically active component (Cline et al., 1986). Phagocytosis is a basic function of this amoeba and one that causes the destruction of cells, whether in cell culture or in human tissue. It is, therefore, intimately involved in pathogenesis. It has been named trogocytosis the piecemeal engulfment of mouse embryo cells by N. fowleri (Marciano-Cabral et al., 1982).It is now known that trogocytosis is accomplished by amoebastomes (Brown, 1979) that the amoebae use to engulf particles of various sizes, including cultivated mammalian cells. In addition to phagocytosis and trogocytosis, N. fowleri seems to injure cultivated mammalian cells by another contact-mediated means (Visvesvara et al., 1990). In contrast, Pettit et al 1996 demonstrated ingestion of whole cells or cell debris by phagocytosis, but, contrary to
- 26 -
the observations described above, concluded that trogocytosis was not involved. These authors and others (Page, 1967 and Diaz et al., 1991)have also demonstrated sucker-like structures termed amoebostomes which are thought to be involved in the engulfment of particles.
In previous reports, the contact-dependent killing mechanisms are operative as well and are likely to involve the ingestion of nerve cell membranes through a ‘food- cup’ structure on the amoeba surface (Cline et al., 1986 and Marciano-Cabral et al., 1983).Furthermore, N. fowleri are highly phagocytic cells and it is likely that they endocytose and destroy nerve cells through a contact-dependent killing mechanism (Visvesvara et al., 1990; Im et al., 2003).
In this study, the Nfa1 protein was mainly located at the tips of the pseudopodia and especially food-cups in N. fowleri trophozoites co-cultured with target cells. At 6hr of cultivation, several red-colored spots which appeared to be fragmented CHO cells debris were shown in N. fowleri trophozoites. It should be reiterated that the Nfa1 protein in the free-living amoeba is necessary for locomotion and phagocytosis. Consequently, trophozoites of N. fowleri destroyed several CHO cells in a time-dependent manner, while activating the Nfa1 protein expression involved in the food-cup formation. In other study, an anti-Nfa1 antibody caused a decreasing effect on the cytotoxicity of N. fowleri trophozoites against CHO cells (Jeong et al., 2004).
Finally, in this study we have elucidated that the Nfa1 protein involved in the food-cup formation plays an important role in phagocytic activity as a cell-contact mechanism in pathogenic N. fowleri.
V. CONCLUSION
In this experiment, by confocal microscopic findings, the Nfa1 protein located on cell surface of N. fowleri trophozoites, especially pseudopodia. The Nfa1 protein in N.
fowleri trophozoites co-cultured with CHO cells located on pseudopodia that
contacted with target cells and in a food-cup that formed as a phagocytic structure. Finally, in this study it was elucidated that the Nfa1 protein is involved in the food-cup formation which play an important role of phagocytic activity as a cell-contact mechanism in pathogenic N. fowleri.
- 28 -
BIBLIOGRAPHY
1. Barnett ND, Kaplan AM, Hopkin RJ, Saubolle MA, Rudinsky MF: Primary amoebic meningoencephalitis with Naegleria fowleri. Pediatric Neurol 15:230 234, 1996
2. Brown T: Observations by immunofluorescence microscopy and electron microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J Med Microbiol 12:363-371, 1979
3. Cho MS, Jung SY, Park S, Kim KH, Kim HI, Sohn S, Kim HJ, Im KI, Shin HJ: Immunological characterizations of a cloned 13.1-kilodalton protein from pathogenic Naegleria fowleri. Clin Diagn Lab Immunol 10:954-959, 2003
4. Cline M, Carchman R, Marciano-Cabral F: Movement of Naegleria fowleri stimulated by mammalian cells in vitro. J Protozool 33:10-13, 1986
5. Culbertson CG: The pathogenicity of soil amoebas. Annu Rev Microbiol 25:231-254, 1971
two strains of Acanthamoeba: scanning electron microscopy study. Int J
Parasitol 21:365-367, 1991
7. Im KI, Shin HJ: Acanthamoeba sohi, n. sp., a pathogenic Korean isolate YM-4 from a freshwater fish. Korean J Parasitol 41: 181-188, 2003
8. John DT: Primary amebic meningoencephalitis and the biology of Naegleria
fowleri. Annu Rev Microbiol 36:101-23, 1982
9. Jeong SY, Kang SY, Lee SC, Song KJ, Im KI, Shin HJ: Decreasing effect of an anti-Nfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic
Naegleria fowleri. Korean J Parasitol 42:35-40, 2004
10. Kollars TM, Wilhelm WE: The occurrence of antibodies to Naegleria species in wild mammals. J Parasitol 82:73-77, 1996
11. Ma, P., Visvesvara, G. S., Martinez, A. J., Theodore, F. H., Daggett, P. M., and Sawyer, T. K: Naegleria and Acanthamoeba infections. Rev Infect Dis 12:490– 513, 1990
12. Marciano-Cabral F, Patterson M, John DT, Bradley SG: Cytopathogenicity of
- 30 -
J Parasitol 68:1110–1116, 1982
13. Marciano-Cabral F and John DT: Cytopathogenicity of Naegleria fowleri for rat neuroblastoma cell cultures: scanning electron microscopy study. Infect Immun 40:1214–1217, 1983
14. Marciano-Cabral F: Biology of Naegleria spp. Microbiol Rev 52:114-133, 1988
15. Page FC: Re-definition of the genus Acanthamoeba with descriptions of three species. J Protozool 14: 709-724, 1967
16. Pettit DAD, Williamson J, Cabral GA. Marciano-Cabral F: In vitro destruction of nerve cell cultures by Acanthamoeba spp. a transmission and scanning electron microscopy study. J Parasitol 82: 769-777, 1996
17. Seidel JS, Harmatz P, Visvesvara GS, Cohen A, Edwards J, Turner J: Successful treatment of primary amebic meningoencephalitis. N Eng J Med 306:346-48, 1982
18. Shin HJ, Cho MS, Jung SY, Kim HI, Park S, Kim HJ, Im KI: Molecular cloning and characterization of a gene encoding a 13.1 kDa antigenic protein of
19. Shin HJ, Im KI,: Pathogenic free-living amoeba in korea. Korean J Parasitol 42: 93-119, 2004
20. Visvesvara GS, Martinez AJ: Protozoa: free-living amebae: Infectious Diseases. D Armstrong and S Cohen (eds). London: Mosby. pp.33.1-33.6, 1999
21. Willaert E: Isolement et culture in vitro des amibes de genre Naegleria. Ann Soc
Belg Med Trop 51: 701-708, 1971
- 32 - - 국문요약 -
표적세포와
표적세포와
표적세포와
표적세포와 혼합배양시
혼합배양시
혼합배양시
혼합배양시 병원성
병원성
병원성 파울러자유아메바에서
병원성
파울러자유아메바에서
파울러자유아메바에서
파울러자유아메바에서
Nfa1
Nfa1
Nfa1
Nfa1 단백질의
단백질의
단백질의 역할
단백질의
역할
역할
역할
아주대학교 대학원의학과 강 수 연 (지도교수 : 신 호 준) 파울러자유아메바 (Naegleria fowleri)는 자연환경 속에서 자유생활을 하는 원충류로서 인체와 실험동물들에서 원발성 아메바성 수막뇌염 (primary amoebic meningoencephalitis)을 일으키는 것으로 알려져 있다. 본 실험에 앞서 파울러 자유아메바의 cDNA로부터 면역 및 감염 혈청을 이용하여immunoscreening을 통해 항원 관련 유전자 (antigen-related gene)로
생각되어지는 nfa1 유전자가 클로닝 되었다. 이 nfa1 유전자는 360bp의 염기서열을 가지고 있었으며, 발현 백터를 이용하여 17kDa의 tag이 결합되어있는 재조합 단백질을 얻었다. 이 항-Nfa1 항체를 이용해서, Nfa1 단백질이 파울러 자유아메바 영양형의 위족에 특이하게 발현 되는 것을 볼 수 있었다. 위족 활동과 연관된 Nfa1 단백질의 아메바의 세포독성과의 연관성을 알아본 결과, 항-Nfa1 항체가 CHO세포에 대한 파울러 자유아메바 영양형의 세포독성을 크게 감소시키는데 영향을 준다는 사실을 확인하였다. 파울러
자유아메바의 병원성에 대해 많은 연구에도 불구하고, 이 생물체의
탐식기능(phagocytosis)과 관련된 단백질은 아직 별로 알려져 있지 않다. 본 연구에서는, Confocal microscope를 이용한 immunofluorescence 분석을
통하여 표적세포와 혼합배양된 파울러 자유아메바의 세포-접촉 기전에서 Nfa1 단백질의 위치와 역할을 관찰하였다. Confocal microscope 관찰 결과, Nfa1
단백질은 파울러 자유아메바의 세포 표면 특히, 위족에 위치하고 있었다. 그리고 표적세포와 혼합배양시 파울러 자유아메바에서의 Nfa1 단백질은 표적세포와 접촉하고 있는 위족에 밀집되어 있었고 특히, 식세포 작용을 하는 구조체로 형성된 food-cup에서 뚜렷한 발현양상을 보여주었다. 따라서 병원성의 파울러 자유아메바의 Nfa1 단백질은 아메바의 위족활동에 중요한 역할을 하며, 특히, 표적세포와의 접촉시, 아메바의 식세포 작용을 하는 food-cup의 형성과 활동에 매우 중요한 역할을 하고 있다는 것을 알 수 있었다. 핵심되는 말 : 파울러 자유아메바, 유전자 클로닝, 재조합 단백질, Nfa1 단백질, Confocal microscope