Evaluation of
18F FDG-PET/CT in Patients with Gastric
Signet Ring Cell Carcinoma : Correlation with GLUT-1
Expression and Histologic Finding
by
Bong-Hoi Choi
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Evaluation of
18F FDG-PET/CT in Patients with Gastric
Signet Ring Cell Carcinoma : Correlation with GLUT-1
Expression and Histologic Finding
by
Bong-Hoi Choi
A Dissertation Submitted to The Graduate School of Ajou University in Partial Fulfillment of the Requirements for the Degree of
Master of Medical Sciences
Supervised by
Joon-Kee Yoon, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Bong-Hoi Choi is approved.
SUPERVISORY COMMITTEE
(Sign)
Sang-Uk Han
(Sign)
Joon-Kee Yoon
(Sign)
Young-Sil An
The Graduate School, Ajou University
June, 25th, 2010
i
ABSTRACT
-Evaluation of
18F FDG-PET/CT in Patients with Gastric Signet Ring
Cell Carcinoma : Correlation with GLUT-1 Expression and
Histologic Finding
Purpose Gastric signet ring cell carcinoma(GSRC) isknown to have low FDG uptake. The
aim of the study was to investigate the relation between FDG uptake andglucose
transporter-1 (GLUT-transporter-1) expression in GSRC patients.
Materials and Methods Forty patients (28 men, mean age 54±12) with histologically
confirmed GSRC who underwent a pre-operative PET/CT were enrolled. The maximum
standardized uptake value (SUVmax) was compared with histological parameters and GLUT-1 expression. GLUT-1 expression was divided into membranous or cytoplasmic group.
Result The SUVmax was significantly higher in membranous group than in cytoplasmic
group (6.06 ± 2.79 vs. 3.67 ± 1.54, p=0.03). Gastric wall invasion, depth of invasion, extent of LN metastasis, overall stage and tumor size were also correlated with SUVmax. On the other hand, age, sex and the presence of distant metastasis were not related with SUVmax. Multivariate analysis revealed that the membranous GLUT-1 expression and the extent of LN metastasis were the independent predictive factor of high FDG uptake in GSRC.
ii
SUVmax predicted the presence of membranous expression of GLUT-1 with moderate specificity (82.6%, cut-off = 4.3).
Conclusion This study demonstrates that high FDG uptake is mediated by membranous
GLUT-1 expression in GSRC.
iii
TABLE OF CONTENTS
ABSTRACT ··· ⅰ
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ··· iv
LIST OF TABLES ··· v
Ⅰ. INTRODUCTION ··· 1
Ⅱ. MATERIALS AND METHODS ··· 3
A. MATERIALS ··· 3
B. METHODS ··· 4
1. FDG PET/CT acquisition and image analysis ··· 4
2. Immunohistochemical analysis ··· 5
3. Statistical analysis ··· 6
Ⅲ . RESULTS ··· 7
1. Patients’ characteristics ··· 7
2. Relation between FDG uptake and clinicopathologic parameters ··· 8
3. Multivariate analysis between FDG uptake and clinicopathologic parameters ·· 16
Ⅳ. DISCUSSION ··· 17
Ⅴ. CONCLUSION ··· 21
REFERENCES ··· 22
iv
LIST OF FIGURES
Fig. 1. Representative PET and immunohistochemical staining images for GSRC
v
LIST OF TABLES
Table 1. Characteristics, Type of Surgical Operation of the Patients ··· 8
Table 2. Comparison between visual FDG Uptake and Clinicopathologic Variables in GSRC ··· 10
Table 3. Relation between semiquantitative analysis and Clinicopathologic Variables in GSRC ··· 11
Table 4. Comparison between GLUT-1 staining and Clinicopathologic Variables in GSRC ··· 13
Table 5. Multivariate Regression Analysis between FDG uptake and Clinopathologic Variables in GSRC ··· 16
- 1 -
Ⅰ. INTRODUCTION
Gastric signet ring cell carcinoma (GSRC) is a histological type defined by the microscopic characteristics described by World Health Organization. The prevalence of GSRC has been reported to vary from 3.4 to 39% (Maehara et al, 1992; Theuer et al,
1999; Antonioli and Goldman, 1982) and, in South Korea, incidences of 12.2% and
8.7% have been reported (Kim et al, 1994; Kim et al, 2004). One group of researchers reported that GSRCs shows a slightly higher predominance of females and younger individuals (Maehara et al, 1992), whereas others have found no gender difference
(Antonioli and Goldman, 1982; Kim et al, 2004; Li et al, 2007; Hyung et al, 2002). From
the prognostic point of view, several authors have demonstrated that GSRC does not have a poorer prognosis than the other types of stomach cancer (Maehara et al, 1992;
Theuer et al, 1999; Kim et al, 2004; Hyung et al, 2002), but the issue remains
controversial. Others have suggested that GSRC of advanced gastric cancer type has a poorer prognosis (Kim et al, 1994; Li et al, 2007).
Positron emission tomography (PET) with 2-[18F]fluoro-2-deoxy-D-glucose (FDG) has been widely used for detecting primary tumors, staging, planning treatment, monitoring after treatment, and for prognostic evaluations (Endo et al,
2006; Schoder et al, 2004). FDG is actively transported into the intracellular space by
- 2 -
hexokinase. Because FDG-6-phosphate is not substrate of tricarboxylic acid cycle, it accumulates within cells. Eventually, it is dephosphorylated by glucose-6-phosphatase, which allows the efflux of FDG to the extracellular space. Most tumors are known to show increased glucose metabolism, which provides energy for cell growth and produces the precursors required for nucleotide and lipid synthesis (Ong et al, 2008; Southworth et al, 2003). In several cancer types, malignant cells exhibit increased FDG uptake mediated by elevated levels of GLUT and hexokinase (De Geus-Oei et al, 2007; Paudyal et al, 2008; Hamada et al, 2008; Shim et al, 2009).
Although several previous studies have reported that GSRCs generally show low FDG uptake (Bhure et al, 2007; Buyyounouski et al, 2005; Yoshioka et al, 2003), but several GSRCs were showed intense FDG uptake of PET/CT in our institution. Furthermore, the cause of this variability in FDG uptake has not been evaluated in GSRC. Accordingly, the aim of this study was to investigate whether FDG uptake on PET/CT is related to GLUT-1 expression in GSRC.
- 3 -
Ⅱ. MATERIALS AND METHODS
A. MATERIALS
A total of 144 consecutive gastric cancer patients underwent a preoperative [18F]FDG PET/CT and surgical excision of primary tumors between December 2003 and October 2008. According to the WHO classification for histologic typing of gastric cancers : signet ring cell carcinoma was defined as “ an adenocarcinoma in which a predominant component (more than 50% of the tumor) is made up of isolated or small groups of malignant cells containing intracytoplasmic mucin” (Watanabe et al, 1990), 83 patients without signet ring cell components and 19 patients of mixed with adenocarcinoma and signet ring cell components (less than 50%) were excluded. After excluding two diabetic patients, 40 patients of signet ring cell carcinoma were enrolled in this study. All information on patient characteristics, endoscopic and histologic findings (Borrmann’s classification, depth of invasion, tumor size, and TNM stage) were collated retrospectively by medical record review. The mean interval between PET/CT and surgery was 4 days. All [18F]FDG PET/CT images were reviewed blindly on a workstation. Patients were allocated to a membranous group and a cytoplasmic group according to immunohistochemical staining results. This study was approved by the institutional review board
(AJIRB-- 4 (AJIRB--
MED-KSP-09-256).
B. METHODS
1. FDG PET/CT acquisition and image analysis
After fasting for least 6 h, patients were administrated 370 MBq of [18F]FDG intravenously. All patients were instructed to rest comfortably for 60 min and urinate before scanning. Whole body PET/CT images were obtained on a Discovery ST scanner (GE Healthcare, Milwaukee, WI, USA). Seven to eight frames (3 min/frame) of emission PET data were acquired in two dimensional mode after a noncontrast CT scan from the base of the skull to the upper thigh (tube rotation time of 1 s per revolution, 120 kV, 60 mA, 7.5 mm per rotation, and acquisition time of 60.9 s for a scan length of 867 mm). Emission PET images were reconstructed using an iterative method (ordered-subsets expectation maximization with 2 iterations and 30 subsets, field of view = 600 mm, slice thickness = 3.27 mm) and attenuation-corrected with noncontrast CT. Standardized uptake values (SUVs) were calculated based on injected dose and body weight. Two nuclear medicine physicians reviewed PET/CT images blindly on an AW workstation (version 4.4) by consensus. Depending on the presence of FDG uptake in primary tumor, visual analysis was performed. For the semiquantitative analysis of FDG uptake, polygonal region of interests (ROIs) were
- 5 -
placed on primary tumors and maximum SUVs (SUVmax) were recorded. When primary tumors on PET/CT images were indistinguishable because of low FDG uptake, ROIs were copied from noncontrast CT images.
2. Immunohistochemical analysis
One representative tumor section containing the highest proportion of malignant cells was chosen for each patient for immunohistochemical staining, which was conducted by a board-certified pathologist (K.J.H). All specimens were fixed with 10% formalin and embedded in a paraffin block. Sections of thickness 4 μm were incubated in an oven for 1 h, deparaffinized with xylene for 20 min, and rehydrated. Immunohistochemical staining was performed using an automated staining system (Autostainer, Lab Vision, Fremont, CA, USA). Sections were then subjected to heat-induced antigen retrieval in citrate buffer (pH 6.0) using a microwave oven at 37℃ for 15 min, and endogenous peroxidase activity was blocked by treating them with 3% hydrogen peroxidase for 5 min. They were then washed with Tris-buffered saline, reacted with Ultra V Block (Thermo Fisher Scientific, Fremont, CA, USA) for 10 min, incubated with primary polyclonal antibody at a dilution of 1:250 for GLUT-1 (ab652; Abcam, Cambridge, UK) for 40 min at 37℃, and treated with primary antibody enhancer (Thermo Fisher Scientific, Fremont) for 15 min and then with
- 6 -
HPR polymer (Thermo Fisher Scientific, Fremont) as secondary antibody for 25 min. After Tris-buffered saline washing, sections were treated diaminobenzidine to visualize brown signal. All sections were then counterstained with hematoxilin-eosin, dehydrated, and mounted. Erythrocytes in tissue sections served as positive controls of GLUT-1 expression. An experienced pathologist (K.J.H) evaluated GLUT-1 staining in the absence of clinical information. According to the location of GLUT-1 staining in signet ring cells, sections were divided into a membranous group and a cytoplasmic group. In sections with both membranous and cytoplasmic staining of GLUT-1, more dominant staining area was selected.
3. Statistical analysis
Statistical analysis was performed using Medcalc (version 9.6.4). Relations between SUVmax and other parameters were evaluated using the Mann-Whitney U test and the Kruskall-Wallis test. ROC curve (Receiver Operating Characteristic curve) analysis was used to determine a cut-off of SUVmax for membranous GLUT-1 expression. Multiple regression analysis was used to indentify independent predictors of FDG uptake. P-values less than 0.05 were considered significant.
- 7 -
Ⅲ . RESULTS
1. Patients’ characteristics
Patients’ characteristics and type of surgery are summarized in Table 1. Subtotal gastrectomy was the major type of surgery in this study (28/40).
Table 1. Characteristics and Type of Surgical Operation of the Patients
Variables Values Number Proportion of FDG-avid GSRC Age N=40 42% 54±12 Sex M:F = 28:12
Type of surgical operation Total gastrectomy Subtotal gastrectomy
12 28
- 8 -
2. Relation between FDG uptake and clinicopathologic parameters
In visual analysis of PET/CT, FDG uptake of primary tumor was observed in 17 patients (42%). In comparison between results of visual analysis and clinicopathologic variables are summarized in Table 2. In visual analysis, FDG uptake was related with gastric wall invasion, extent of LN metastasis and primary tumor size. Results of univariate analysis between SUVmax and other clinicopathologic parameters including GLUT-1 expression are summarized in Table 2. Mean SUVmax in the membranous group was significantly higher than in the cytoplasmic group (6.06 ± 2.79 vs. 3.67 ± 1.54, p=0.03, Fig. 1). Of the histologic parameters, gastric wall invasion, T stage, extent of LN metastasis, and tumor size were found to be significantly related to FDG uptake. Advanced gastric cancer (AGC) had significantly higher FDG uptake than early gastric cancer (EGC). SUVmax was found to increase significantly with T stage (T3-4 vs. T1-2; 3.60 ± 2.19 vs. 5.58 ± 2.96, p=0.0019) and N stage (N2-3 vs. N0-1; 6.63 ± 3.40 vs. 3.39 ± 1.13, p=0.0002). GSRCs with their size > 3cm showed higher FDG uptake than those ≤ 3 cm. Age, sex and the presence of distant metastasis were not found to be related to FDG uptake. Considering immunohistochemical staining of GLUT-1, the difference between membrane group and cytoplasm group were summarized at the Table 4.
- 9 -
Table 2. Comparison between visual FDG Uptake and Clinicopathologic Variables in GSRC Clinicopathologic features FDG uptake (+) n = 17 FDG uptake (-) n = 23 P-value Age <55 8 13 ≥55 9 10 0.78 Sex Male 14 14 Female 3 9 0.26
Gastric wall invasion
AGC 17 13 EGC 0 10 0.0056† Borrmann type II 1 III 10 7 IV 6 6 0.60 Depth of invasion T1 0 9 T2 3 6 T3 14 5 T4 0 3 0.0008† Extent of LN metastasis
- 10 - N0 1 15 N1 5 3 N2 3 2 N3 8 3 0.0021† Distant metastasis Negative 15 21 Positive 2 2 0.79 Tumor Size < 3 cm 0 9 ≥ 3cm 17 14 0.01† GLUT-1 expression Membranous 10 7 Cytoplasmic 7 16 0.14
P-value were evaluated using †Chi-square test. GSRC = gastric signet ring cell carcinoma, AGC = advanced gastric cancer, EGC = early gastric cancer, GLUT-1 = glucose transporter-1.
- 11 -
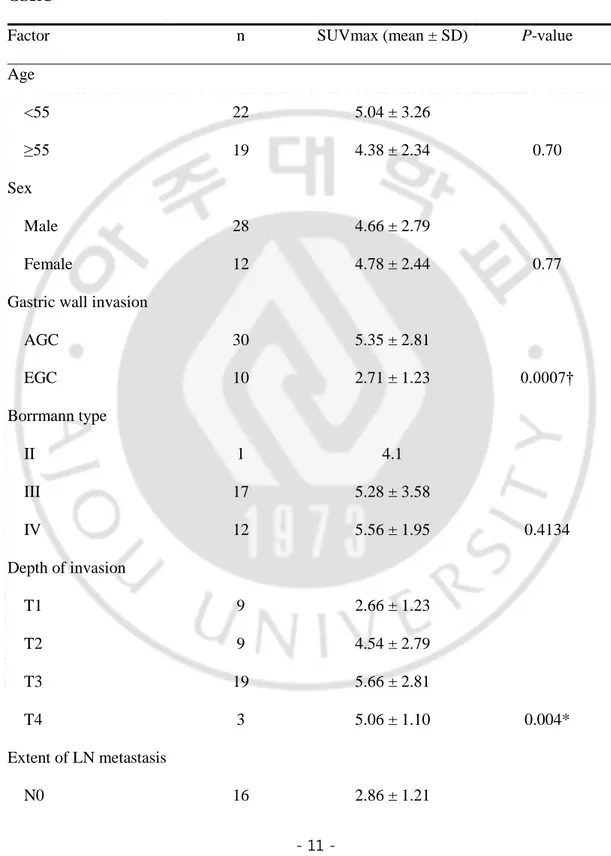
Table 3. Relation between semiquantitative analysis and Clinicopathologic Variables in GSRC
Factor n SUVmax (mean ± SD) P-value
Age <55 22 5.04 ± 3.26 ≥55 19 4.38 ± 2.34 0.70 Sex Male 28 4.66 ± 2.79 Female 12 4.78 ± 2.44 0.77
Gastric wall invasion
AGC 30 5.35 ± 2.81 EGC 10 2.71 ± 1.23 0.0007† Borrmann type II 1 4.1 III 17 5.28 ± 3.58 IV 12 5.56 ± 1.95 0.4134 Depth of invasion T1 9 2.66 ± 1.23 T2 9 4.54 ± 2.79 T3 19 5.66 ± 2.81 T4 3 5.06 ± 1.10 0.004* Extent of LN metastasis N0 16 2.86 ± 1.21
- 12 - N1 8 4.16 ± 1.19 N2 5 6.7 ± 2.78 N3 11 6.6 ± 2.39 0.006* Distant metastasis Negative 36 4.78 ± 2.82 Positive 4 3.95 ± 2.84 0.91 Tumor Size < 3 cm 10 2.2 ± 0.48 ≥ 3cm 30 8.03 ± 3.69 0.0001† GLUT-1 expression Membranous 17 6.06 ± 2.79 Cytoplasmic 23 3.67 ± 1.54 0.03†
P-value were evaluated using *Kruskal-Wallis and †Mann-Whitney U test. GSRC = gastric signet ring cell carcinoma, SUVmax = maximum standardized uptake value, AGC = advanced gastric cancer, EGC = early gastric cancer, GLUT-1 = glucose transporter-1.
- 13 -
Table 4. Comparison between GLUT-1 staining and Clinicopathologic Variables in GSRC Clinicopathologic features Membrane n = 17 Cytoplasm n = 23 P-value Age <55 6 15 ≥55 11 8 0.12 Sex Male 13 15 Female 4 8 0.67
Gastric wall invasion
AGC 13 17 EGC 4 6 0.85 Borrmann type II 0 1 III 10 7 IV 3 9 0.13 Depth of invasion T1 3 6 T2 5 4 T3 8 11 T4 1 2 0.79 Extent of LN metastasis
- 14 - N0 5 11 N1 4 4 N2 3 2 N3 5 6 0.64 Distant metastasis Negative 17 19 Positive 0 4 0.20 Tumor Size < 3 cm 4 5 ≥ 3cm 13 18 0.80 SUVmax 6.06 ± 2.79 3.67 ± 1.54 0.03*
P-value were evaluated using †Chi-square test and *Mann-Whitney U test. AGC = advanced gastric cancer, EGC = early gastric cancer, GLUT-1 = glucose transporter-1.
- 15 -
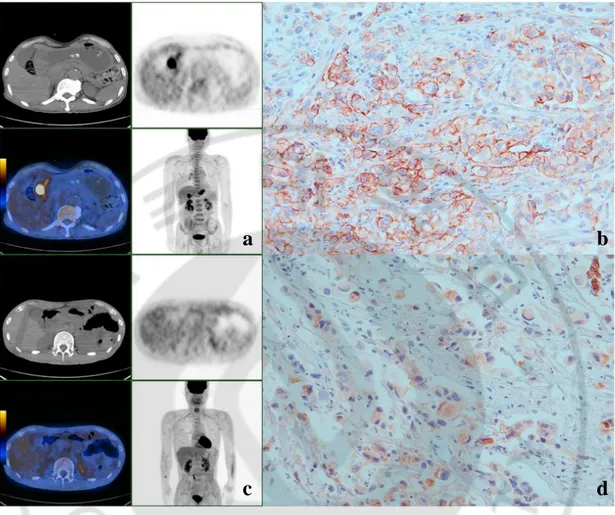
Fig. 1. Representative PET and immunohistochemical staining images for GSRC with GLUT-1 expression on membranes and cytoplasms. (a, b) A case of stage IV GSRC
showing intense FDG uptake (SUVmax 8.4) in the gastric antrum (arrow) and membranous GLUT-1 staining. (c, d) Another case of stage IV GSRC showing no significance FDG uptake in gastric wall and cytoplasmic GLUT-1 staining (original magnification ⅹ400).
a
b
- 16 -
3. Multivariate analysis between FDG uptake and clinicopathologic parameters
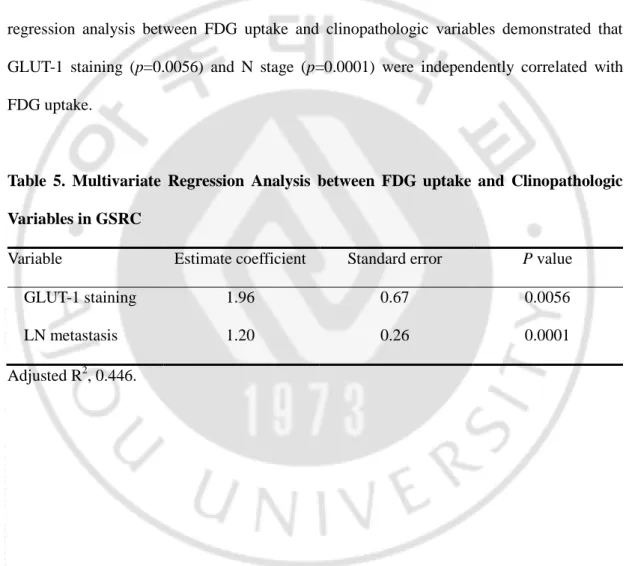
Multivariate analysis was performed between SUVmax and significantly correlative clinopathologic factors (gastric wall invasion, T stage, the extent of LN metastasis, stage, tumor size and GLUT-1 expression) in univariate analysis. As shown in Table 3, multivariate regression analysis between FDG uptake and clinopathologic variables demonstrated that GLUT-1 staining (p=0.0056) and N stage (p=0.0001) were independently correlated with FDG uptake.
Table 5. Multivariate Regression Analysis between FDG uptake and Clinopathologic Variables in GSRC
Variable Estimate coefficient Standard error P value
GLUT-1 staining 1.96 0.67 0.0056
LN metastasis 1.20 0.26 0.0001
- 17 -
Ⅳ. DISCUSSION
In this study, the relation between the clinicopathologic findings of GSRC including GLUT-1 expression, and FDG uptake on PET/CT images was evaluated. It was found that 42.5% of GSRCs showed membranous GLUT-1 expression, and these GSRCs had significantly higher SUVmax values than those showing cytoplasmic expression. This result suggests that a considerable proportion of GSRCs show elevated FDG uptake due to the membranous expression of GLUT-1. The majority of previous studies evaluated this association in all types of gastric cancer, and not specifically in GSRC.
Degree of GLUT-1 expression has been previously shown to be correlate with increased FDG uptake in a variety of human malignancies (De Geus-Oei et al, 2007;
Paudyal et al, 2008; Hamada et al, 2008; Shim et al, 2009). Similarly, in gastric carcinoma,
GLUT-1 expression has been shown to be significantly correlated with FDG uptake (p=0.002). Median SUVmax has been reported to be higher in gastric cancers with detectable GLUT-1 expression (6.9, range 2.3-14.1) than in those without GLUT-1 expression (3.1, range 1-8.8) (Alakus et al, 2010). The above mentioned results could explain the variability of FDG uptake in gastric cancer during initial staging.
Previous studies reported that non-intestinal type gastric cancers, including GSRC, show lower FDG uptake than the intestinal type. Stahl et al. reported that 9 of
- 18 -
22 non-intestinal type gastric cancers (42%) and 15 of 18 intestinal type (83%) showed detectable FDG uptake by visual analysis (Stahl et al, 2003). In another study by Yamada et al., non-cohesive type gastric cancer was found to show significantly lower GLUT-1 expression and FDG uptake than the cohesive type
(Yamada et al, 2006). De Potter et al. also reported that only 25% of GSRCs had FDG
avidity in a small group study (n=8) (De potter et al, 2002), which concurs with that found in the Korean population (Han et al, 2009). Han et al. concluded that the intestinal type has significantly higher SUVmax values than the non-intestinal type
(Han et al, 2009). In addition, Kawamura et al. found that the expression of GLUT-I
was higher in papillary and tubular adenocarcinoma than in signet ring cell and mucinous adenocarcinoma (Kawamura et al, 2001). However, in one recent study, 78% of GSRCs (7/9) were included in an FDG-avid group (Mochiki et al, 2004). In the present study, 42% of GSRCs had detectable FDG uptake on PET by visual analysis, and GSRCs with membranous GLUT-1 expression showed significantly higher FDG uptakes than those with cytoplasmic expression, which suggests that degree of FDG uptake is affected by GLUT-1 expression on signet ring cells. These results are consistent with those of previous studies that have examined the nature of relation between FDG uptake and GLUT-1 expression in gastric cancer.
In addition to GLUT-1 expression, a number of histologic factors were also found to be significantly related to FDG uptake. In particular, primary tumor size of GSRC was found to be significantly associated with SUVmax, which concurs with the
- 19 -
results of earlier studies (Stahl et al, 2003; Han et al, 2009: Mochiki et al, 2004; Yamada et
al, 2006; Chen et al, 2005). Chen et al reported that FDG uptake was significantly
different in EGCs and AGCs (Chen et al, 2005), which concurs with results of present study. Furthermore, in the present study, depth of invasion (T stage), regional LN involvement (N stage), and overall GSRC stage were found to be significantly related to primary tumor FDG uptake, which also closely agrees with the results of previous studies (Mochiki et al, 2004; Chen et al, 2005) and suggest that FDG uptake is closely associated with tumor progression in GSRC. On the other hand, these results indicate that the diagnostic ability of FDG PET in signet ring cell type EGC is likely to be lower than in signet ring cell type AGC.
In the present study, multiple regression analysis showed that GLUT-1 staining and regional lymph node involvement were independently related to FDG uptake, which concurs with a previous report issued by Yamada et al, in which GLUT-1 expression was found to be the factor that influenced FDG uptake in gastric carcinoma (Yamada et al, 2006).
This study has several limitations that require consideration. First, it was based on a retrospective analysis performed at a single institution and some of the patients with GSRC did not undergo surgery because of advanced stage disease. Accordingly, caution should be exercised when generalizing findings of present study. Second, physiologic FDG uptake on PET images was often showed in gastric mucosa. In the present study, the water-induced gastric distention method to determine primary
- 20 -
gastric tumor FDG uptake in about one third of our patients was adapted, but the analysis was conducted using non-distended PET images for homogeneity reasons. This limitation warrant a future prospective study incorporating the water distention protocol. Third, as FDG uptake is specifically regulated by type-II-hexokinase and other glucose transporters, their correlation with FDG uptake in GSRC is also worthy of further investigation.
- 21 -
Ⅴ. CONCLUSION
In conclusion, the FDG uptake in primary tumor of GSRC is variable and is assumed to be mediated by the membranous expression of GLUT-1. Future studies concerning the expression of hexokinase and other glucose transporters will be required to ascertain the contribution of GLUT-1 to FDG uptake in GSRC.
- 22 -
REFERENCES
1. Alakus H, Batur M, Schmidt M, Drebber U, Baldus SE, Vallböhmer D, Prenzel KL, Metzger R, Bollschweiler E, Hölscher AH, Mönig SP Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun 31:532-538, 2010
2. Antonioli DA and Goldman H: Changes in the location and type of gastric adenocarcinoma. Cancer 50:775-781, 1982
3. Bhure U, Schmitt AM, Pestalozzi BC, Hany TF, Strobel K FDG-negative signet ring cell cancer of the stomach with FDG-positive skin metastases. Clin Nucl Med 32:226-228, 2007
4. Buyyounouski MK, Klump WJ, Konski A, Wu H, Adler LP FDG PET imaging of signet-ring cell adenocarcinoma of the stomach. Clin Nucl Med 30:118-119, 2005
5. Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 103:2383-2390, 2005
6. de Geus-Oei LF, van Krieken JH, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer 55:79-87, 2007
7. De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging 29:525-529, 2002
- 23 -
8. Endo K, Oriuchi N, Higuchi T, Iida Y, Hanaoka H, Miyakubo M, et al: PET and PET/CT using 18F-FDG in the diagnosis and management of cancer patients. Int J Clin Oncol 11:286-296, 2006
9. Hamada K, Tomita Y, Qiu Y, Zhang B, Ueda T, Myoui A, et al. 18F-FDG-PET of musculoskeletal tumors: a correlation with the expression of glucose transporter 1 and hexokinase II. Ann Nucl Med 22:699-705, 2008
10. Han EJ, Choi WH, Chung YA, Kim KJ, Maeng LS, Sohn KM, et al. Comparison between FDG uptake and Clinicopathologic and Immunohistochemical Parameters in Pre-operative PET/CT Scan of Primary Gastric Carcinoma. Nucl Med Mol Imaging 43:26-34, 2009
11. Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, et al: Early gastric carcinoma with signet ring cell histology. Cancer 94:78-83, 2002
12. Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer 92:634-641, 2001
13. Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, et al: Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg 74:1060-1064, 2004
14. Kim JP, Kim SC, Yang HK: Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol 3:221-227, 1994
15. Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, et al: Advanced gastric carcinoma with signet ring cell histology. Oncology 72:64-68, 2007
- 24 -
16. Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, et al: Signet ring cell carcinoma of the stomach. Cancer 69:1645-1650, 1992
17. Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg 28:247-253, 2004
18. Ong LC, Jin Y, Song IC, Yu S, Zhang K, Chow PK 2-[18F]-2-deoxy-D-glucose (FDG) uptake in human tumor cells is related to the expression of GLUT-1 and hexokinase II. Acta Radiol 49:1145-1153, 2008
19. Paudyal B, Oriuchi N, Paudyal P, Higuchi T, Nakajima T, Endo K Expression of glucose transporters and hexokinase II in cholangiocellular carcinoma compared using [18F]-2-fluro-2-deoxy-D-glucose positron emission tomography. Cancer Sci 99:260-266, 2008
20. Schoder H, Larson SM, Yeung HW PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl Med 45:72-81, 2004
21. Shim HK, Lee WW, Park SY, Kim H, So Y, Kim SE Expressions of glucose transporter Types 1 and 3 and hexokinase-II in diffuse large B-cell lymphoma and other B-cell non-Hodgkin's lymphomas. Nucl Med Biol 36:191-197, 2009
22. Southworth R, Parry CR, Parkes HG, Medina RA, Garlick PB Tissue-specific differences in 2-fluoro-2-deoxyglucose metabolism beyond FDG-6-P: a 19F NMR spectroscopy study in the rat. NMR Biomed 16:494-502, 2003
- 25 -
23. Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 30:288-295, 2003
24. Theuer CP, Nastanski F, Brewster WR, Butler JA, Anton-Culver H: Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Am Surg 65:915-921, 1999
25. Watanabe H, Jass JR, Sobin LH. Histological typing of oesophageal and gastric tumours. WHO international histological classification of tumors. 2nd ed. Berlin, Springer-Verlag, 1990
26. Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med 20:597-604, 2006
27. Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med 44:690-699, 2003
- 26 - - 국문요약 -
원발성 위반지세포암종 환자의 수술 전
18F FDG 섭취와
GLUT-1 발현 정도와 조직학적 소견과의 비교연구
아주대학교 대학원의학과 최 봉 회 (지도교수: 윤 준 기) 연구목적 : 이전의 몇몇 연구들에서 위 반지세포암은 낮은 FDG 섭취를 보이는 것으로 알려져 있다.이에 본 연구에서는 위 반지암 환자들에서 FDG 섭취정도와 1형 당전달체 발현과의 연관성을 조사하고자 하였다. 재료 및 방법 : 위 절제술후 병리학적으로 진단된 위 반지세포암 환자 40명(남 자 28명, 평균 연령 54세)을 대상으로 하였다. 위 절제술을 시행하기 전 실시한 PET/CT에서 측정된 원발암종부위의 FDG 섭취정도(SUVmax)와 조직학적 인 자 및 1형 당전달체 발현정도와의 관련성을 조사하였다. 원발 암종의 1형 당전 달체의 발현부위에 따라 세포막형과 세포질형으로 구분하였다.- 27 - 결과 : 위 반지세포암의 1형 당전달체 세포막 발현군에서 세포질 발현군보다 유 의하게 높은 FDG 섭취를 보였다(6.06 ± 2.79 vs. 3.67 ± 1.54, p=0.03). 위 반지세포암의 위벽 침윤정도, 임파절 전이정도, 병기 및 원발암종의 크기 등은 SUVmax와 유의한 관련성을 보였으나, 환자의 나이 및 성별, 원격전이 여부 등 은 FDG 섭취정도와 관련을 보이지 않았다. 다변량분석에 따르면, 위 반지세포암 에서 높은 FDG 섭취와 유의한 연관성을 가지는 독립적인 인자는 1형 당전달체 의 세포막 발현과 림프절 전이정도로 나타났다. 위 반지세포암의 FDG 섭취정도 는 1형 당전달체의 세포막 발현을 예측하는데 있어서 중등도의 특이도를 가지는 것으로 나타났다(82.6%, cut-off = 4.3). 결론 : 위 반지세포암의 FDG 섭취정도는 다양하게 나타날 수 있으며, 1형 전달 체의 세포막 발현유무가 높은 FDG 섭취와 유의한 관련이 있었다. FDG 섭취정 도에 영향의 미칠 수 있는 다른 당전달체 및 hexokinase의 발현정도와의 관련 성에 대한 후속 연구가 필요할 것으로 보인다. 핵심어 : 위반지세포암, FDG, 당전달체