관련 문서
other new business models; welcomes, in particular, the development of commercial models that separate consumption from material ownership, in which the function of the
DNA-based testing includes pre- and postnatal genetic testing for the diagnosis of genetic diseases, carrier testing for genetic diseases, susceptibility genetic testing
Human Resources Development (HRD) has been the most important factor in Korea’s escape from vicious cycle of poverty and underdevelopment that had existed for
The Development and Application of Unified Prestress System with Post-tension and Post-compression for Bridge... Unified Prestress
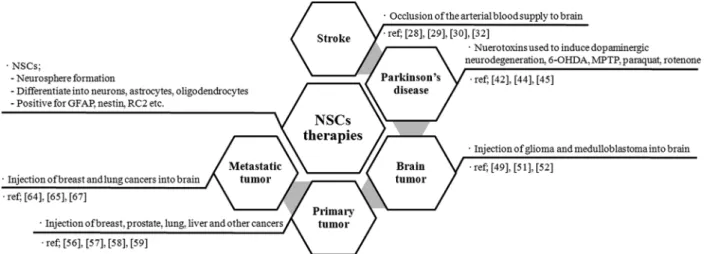
– Embryonic stem cell : Pluripotent cells that can give rise to all tissue types. – Adult stem cell : Multipotent cells
In addition, Korea enacted International Development Cooperation Act(enforcing in July 2010)as basic law of ODA and is seeking for various measures for the performance
In this study, we investigated the effects of low-power CO 2 laser on proliferation on human gingival fibroblast cells so that determine laser
Determining optimal surface roughness of TiO₂blasted titanium implant material for attachment, proliferation and differentiation of cells derived from