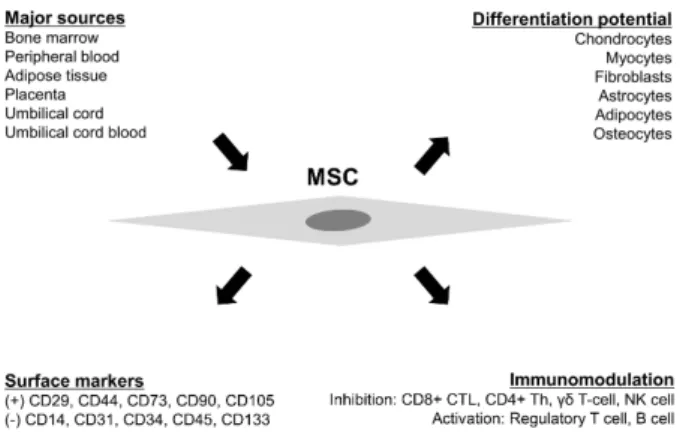
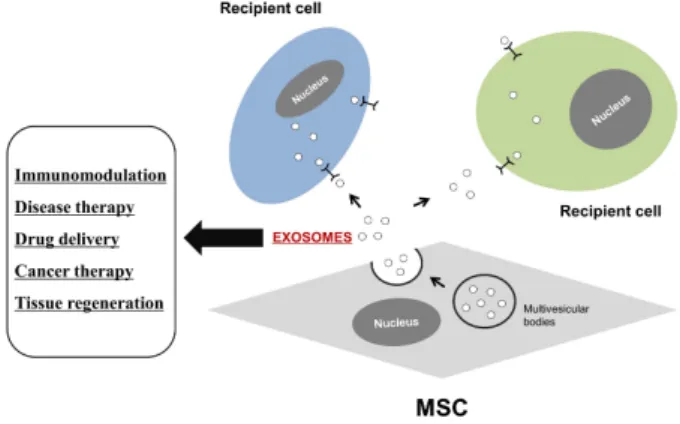
관련 문서
The study derived the problems according to results of field-centered synthetic analysis on yellow croakers of fishery system currently used in Chujado, Korea
The purpose of this study was to evaluate the effect of these substances on bone regeneration by applying these materials to bone defects after cyst
Our results also showed that pigment epithelium-derived factor concentration in vitreous was higher than that in serum, suggesting that it is mainly derived from
In this Thesis, an empirical study on the Influence of Consumer's Preferences and Satisfaction on Purchase Intention of Korean Automobile in Vietnamese Consumers
Hesperetin belongs to the class of flavonoids, called flavanones, which are abundant in Citrus fruits. Hesperetin is derived from the hydrolysis of its
This study derived the financial risk elements of the sports circle and suggested the methods to decrease subsidy support and increase financial independence
In this study, platelet-derived growth factors-BB (PDGF-BB), vascular endothelial growth factor (VEGF), and insulin-like growth factor-1 (IGF-1) were quantified in the PRF
– Water-filling solution in the frequency-time domain is derived – Theoretical limit of data rate in single user OFDM. • Transmit power allocation