Hypercholesterolemia and Coronary Plaque Composition in Patients with Coronary Artery Disease: A Virtual Histology - Intravascular Ultrasound Study
전체 글
수치
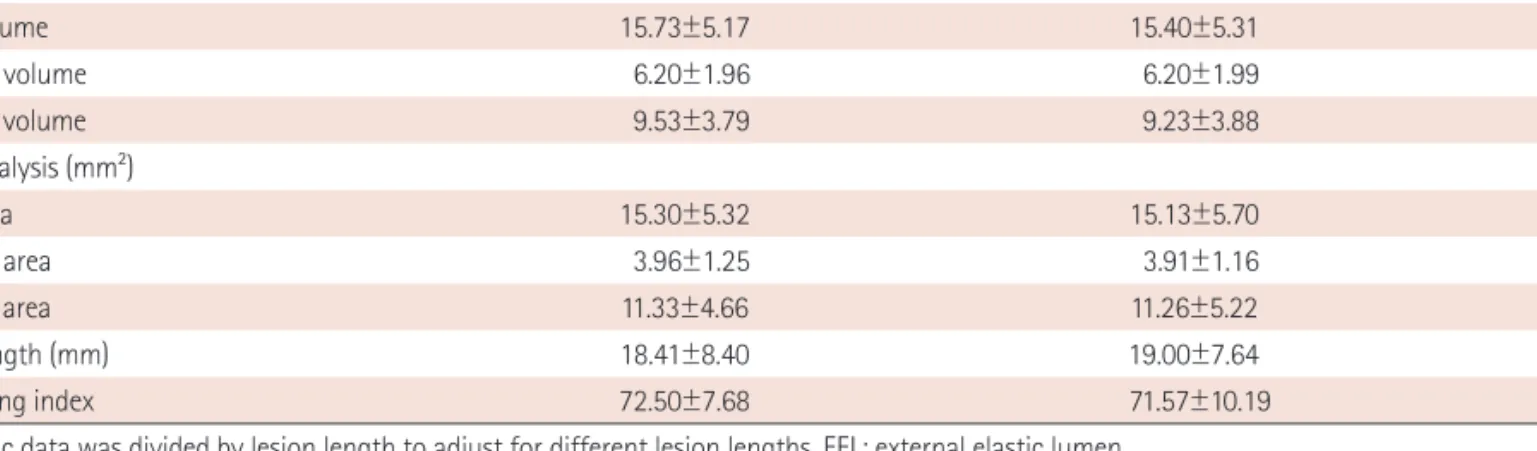
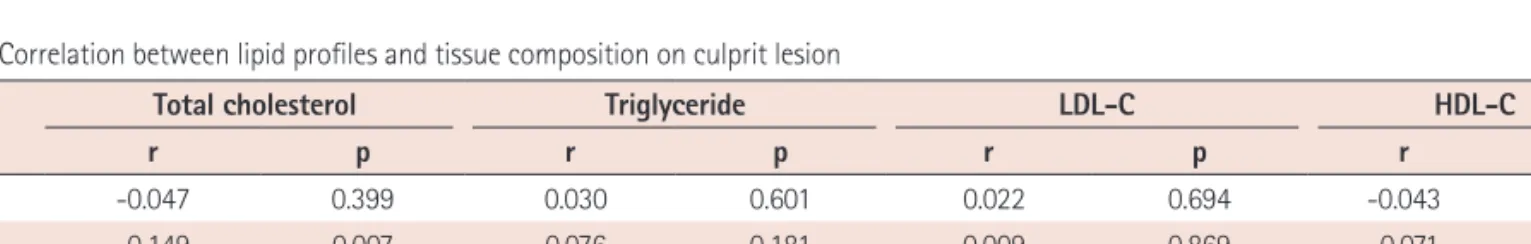
관련 문서
In addition, a recent study reported that anxiety and depression were associated with increased medical costs in general medical inpatient suggesting that anxiety
Thus, this study investigated seasonal variation and species composition of the fish fauna associated with drifting seaweed present in coastal waters
In this study, we investigated the effects of a HF+HS diet in DMM-induced OA mice and found that compared with a HF diet, a HF+HS diet promoted OA. Moreover, our results
Incident major cardiovascular events (coronary artery disease, ischemic stroke, hemorrhagic stroke and cardiovascular mortality) were set as primary end points.
In our study, however, change in abdominal visceral fat remained associated with future pulse pressure after adjusting for HOMA-IR and fasting plasma glucose, implying
Among the various pulmonary manifestations, interstitial lung disease (ILD) is known to be associated with substantial morbidity and mortality rates in RA patients.. As RA-ILD
Our study shows a significant difference in the incidence of VUR among children with and without renal lesions (42.9% vs. According to the systematic overview of 33 studies
Although visceral fat adiposity has known to be associated with clinical, pathologic, and oncologic outcomes in patients with colorectal cancer (CRC), the