31
책임저자:최동호, 서울시 용산구 한남동 657
순천향대학교 의과대학 외과학교실, 140-743 Tel: 02-709-9490, Fax: 02-709-1682
E-mail: dhchoi@hosp.sch.ac.kr
This work was supported by the grants from The Korean Society for Transplantation and Stem Cell Research, 21C Frontier R&D Program, Republic of Korea (SC3210).
로의 의미를 가진다.
(대한이식학회지 2007;21:31-37)
중심 단어: 인간 제대혈, 인슐린 생산세포, Islet 생체 외에서의 인간 제대혈 줄기세포의 인슐린 생
산세포로의 분화
순천향대학교병원
1줄기세포치료센터,
2임상분자생물
학연구소,
3외과,
4종양혈액내과,
5내분비내과
정희정1,2․김숙자1,2․임미선1,3․원종호1,2,4․박형규1,5․
최동호1,3
목적: 췌장의 islet에 있는 베타세포의 자동면역파괴에 의 해 발생하는 당뇨병의 경우 인슐린의 보충이 필요하다. 췌 장의 islet 세포의 이식이 가장 효과적인 방법이지만 공여 자의 부족이라는 문제점이 있다. 이를 해결하기 위해 많은 대체원천들에 관한 연구가 진행되고 있는데 이 논문에서 는 그 중 인간 제대혈로부터 인슐린 생산세포를 생체 외 에서 분화 유도하였다. 방법: 인간 제대혈로부터 단핵구 세포를 분리하고 3×10
6/cm
2의 세포를 0.006% 콜라겐 코 팅된 배양접시에서 1% DMSO가 첨가된 serum-free low DMEM (0.5 mM glucose)에서 3일간 배양하였다. 10% FBS 가 포함된 high DMEM (25 mM glucose) 배양액으로 교체 하고 7일간 더 배양하였다. 이것이 정확한 인슐린 생산세 포인지를 확인하기 위하여 10일째에 immunohistochemistry 로 insulin과 C-peptide 염색하였다. 결과: 인간 제대혈에서 기원된 세포들을 생체 외에서 인슐린 생산세포로 분화 유 도 시 islets와 유사한 cluster형태를 띄게 되는데 7일째부터 관찰되었으며 10일째 cluster를 형성한 인슐린 생산세포는 insulin과 C-peptide를 발현하였다. 결론: 인간 제대혈로부 터 인슐린 생산세포를 분화 유도함으로써 기존의 이식할 islet의 부족이라는 문제점을 해결하고 당뇨병 치료를 가 능하게 하는 세포치료법의 가능성을 제시하는 기초자료
Human Umbilical Cord Blood-derived Cells Generate Insulin-producing Cells In Vitro
1Stem Cell Therapy Center, Soonchunhyang University Hospital, 2Institute for Clinical Molecular Biology Research, Soonchunhyang University College of Medicine, Departments of 3Surgery, 4 Hematology-Oncology and 5Endocrinology, College of Medicine, Soon- chunhyang University, Seoul, Korea
Hee Jeong Cheong, M.S.
1,2, Sook Ja Kim
1,2, Mi Sun Lim
1,3, Jong Ho Won, M.D., Ph.D.
1,2,4, Hyeong Kyu Park, M.D., Ph.D.
1,5and Dongho Choi, M.D., Ph.D.
1,3INTRODUCTION
Pancreas and kidney organ transplantations have been per- formed for patients with diabetes-induced renal insufficien- cy.(1,2) For less invasive surgical modalities, pancreatic islet transplantation has been developed.(3-5) Edmonton protocols, which were characterized by the infusion of multiple, fresh donor islets followed by the host immune suppression with nonsteroidal regimens, have been reported to improve long-term graft acceptance.(6) In both whole pancreas and islet trans- plantations, however, the lack of donor tissues has to be re- solved. Most studies have made an effort to extend pancreatic islets and to find new pancreatic islet sources. Recent studies showed embryonic stem cells (ES cells),(7,8) pancreatic ductal cells,(9,10) hepatic stem cells,(11) and bone marrow (BM)-de- rived cells,(12,13) as alternative sources of IPCs.
Here, we showed that hUCB-derived cells, when cultured under defined conditions, can be efficiently induced to dif- ferentiate into IPCs in vitro. Furthermore, these cells self-as- semble to form pancreatic islet-like structures. We confirmed these clusters were positive for insulin and C-peptide by immunohistochemistry.
METHODS
1) Isolation of MNCs from hUCBWe isolated MNCs from hUCB by a conventional centrifuge
method through a Ficoll-density gradient. In brief, a whole-
Table 1. Primer for RT-PCR
Gene Sequence (5'-3') Product size (bp)
Nkx6.1-F CTC CTC CTC GTC CTC GTC GTC GTC 700
Nkx6.1-R CTT GAC CTG ACT CTC TGT CAT C
Ngn3-F ACT GAG CAA GCA GCG ACG GAG TC 448
Ngn3-R GCA CCC ACA GCC CAG CGA CAG AC
Pax4-F GTG GGC AGT ATC CTG ATT CAG T 308
Pax4-R TGT CAC TCA GAC ACC TTT CTG G
Pax6-F CCG AGA GTA GCG ACT CCA G 239
Pax6-R CTT CCG GTC TGC CCG TTC
β-actin-F GTGGGGCGCCCCAGGCACCA 536
β-actin-R CTCCTTAATGTCACGCACGATTTC
Nkx6.1 = Nk6, transcription factor related locus 1; Ngn3 = neurogenin 3; Pax4, 6 = paired box gene 4, 6.
blood sample was layered onto the top of Ficoll (HISTOPAQUE- 1077, SIGMA-ALDRICH, MO, USA), which was followed by a centrifugation at 2,500 rpm for 30 minutes (min). MNCs were recovered and washed twice with phosphate-buffered saline (PBS). Five samples of hUCB were used for this test.
2) hUCB MNCs culture
The hUCB MNCs were cultured in serum-free low (5.5 mM glucose) Dulbecco's modified Eagle's medium (DMEM, GIBCO, NY, USA) at a cell density of 3×10
6/cm
2in the presence of 1% dimethyl sulphoxide (DMSO, SIGMA) for 3 days followed by high (25 mM glucose) DMEM (GIBCO) supplemented with 10% fetal bovine serum (FBS, GIBCO) for 7 additional days at 95% humidified 37
oC, CO
2incubator. They were plated in plastic six well plates on slide coverslips (22×22 mm
2) coated with 0.006% type I collagen, which was extracted from the rat-tail tendon by the method described by Michalopoulos and Pitot.(14)
3) Immunofluorescent staining
Trans-differentiated hUCB-derived cells were grown on coated coverslips until cluster formed at approximately 10 days of culture. Coverslips were fixed in a 4% paraformaldehyde/
PBS solution at room temperature (RT) for 15 min; then the cells were permeabilized for 30 min at -20
oC with methanol.
The coverslips were further treated with a 5% skim milk in TBS-Ca blocking medium for 1 hour (h) at RT. The coverslips were treated with the primary antibody: rabbit anti-human insulin (Santa Cruz, CA, USA) and rabbit anti-human C-peptide (LINCO, Mo, USA) overnight at 4
oC (which had been diluted at 1:100 with the blocking medium). After washing with
TBS-Ca, the coverslips were incubated at RT for 2 h with secondary antibody: Cy3-conjugated affinipure goat anti- rabbit IgG (Jackson Immuno Research, Pa, USA). DAPI (Vector Lab, CA, USA) was used for nuclear staining. The cells were then observed under a fluorescent microscope.
4) RT-PCR (reverse transcription-polymerase chain reaction)
By RT-PCR analysis, we examined the expression of
Nkx6.1, NGN3, Pax4, and Pax6 gene and beta-actin (β-act) as
a internal control. Total RNA was isolated from uncultured
hUCB MNCs, hUCB derived-cluster cultures, and human islets
by the RNeasy kit (QIAGEN, Germany). One microgram of
total RNA was reverse transcribed using 400 units Moloney
Murine Leukemia Virus reverse transcriptase (M-MLV RT,
Promega, WI, USA) in a 50 uL volume containing 40 pmole
oligo- dT
20primer, 50 mM deoxynucleotide triphosphate
(dNTPs, Applied Biosystems, CA, USA), 50 units recombinant
RNasin ribonuclease inhibitor (Promega), and buffers supplied
by the manufacturer for 60 min at 42
oC. For PCR, 5 uL of
cDNA was included in a total of 50 uL reaction mixture,
containing each 20 pmole forward and reverse primers, 40 mM
dNTPs, buffers supplied by the manufacturer, and 5 units Taq
DNA Polymerase (Roche, IN, USA). Primers were as
mentioned in Table 1. PCR analysis performed in an automated
iCycler thermal cycle (BIO-RAD, USA). The thermal cycling
protocol began with a denaturing step of 95
oC for 5 min, then
35 cycles of 94
oC 1 min, 58
oC 1min, 72
oC 1 min, and finished
with 72
oC for 10 min. PCR products were run on 2% agarose
gels and stained with ethidium bromide. The running gels were
captured using the Gel Doc 2000 Image Analysis System
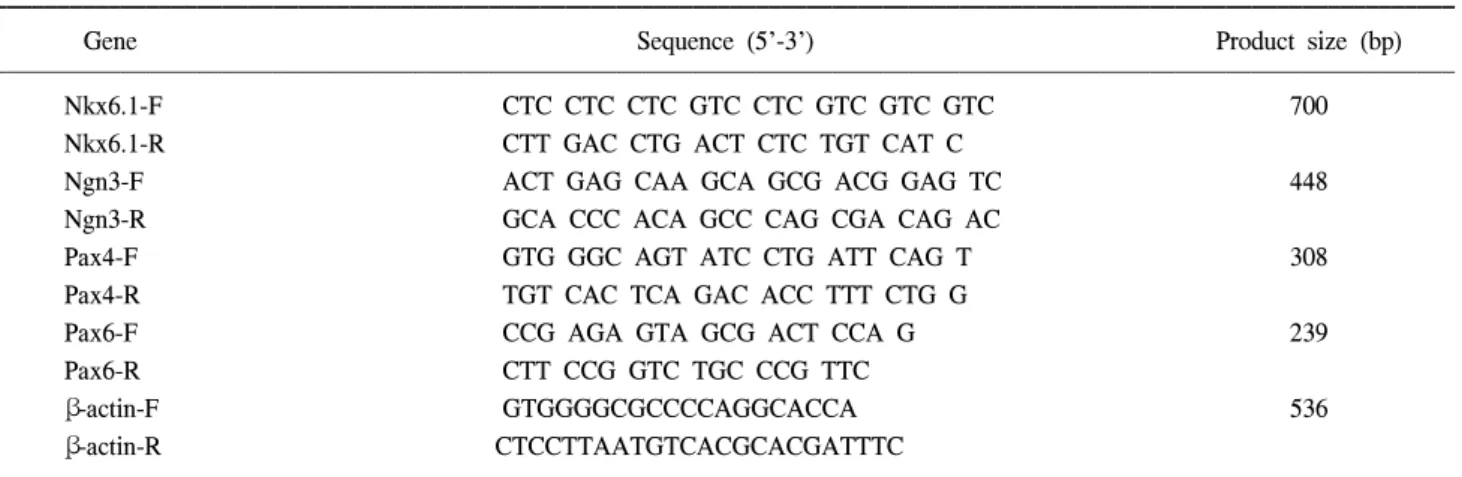
Fig. 1. hUCB cells differentiated into pancreatic-like cells. (A) The hUCB MNCs were plated on 0.006% rat-tail collagen (RTC) coated coverslips contained within plastic six well culture dishes. (B) Clusters similar to islets were formed when The hUCB MNCs were cultured in serum-free low DMEM in the presence of 1% DMSO for 3 days followed by high DMEM supplemented with 10% FBS for 7 additional days. (C) Human islets extracted from normal pancreas. (D) Multiple clusters can be seen in single field (×10). (E, F) At higher magnifications they appear to have defined edges and structure (×20, ×40).
(BIO-RAD).
RESULTS
1) hUCB-derived cells trans-differentiation into IPCs
The hUCB MNCs were plated on 0.3% rattail collagen (RTC) coated coverslips contained within plastic six well culture dishes. The hUCB MNCs were cultured in serum-free low DMEM at a cell density of 3×10
6/cm
2in the presence of 1% DMSO for 3 days followed by high DMEM supplemented with 10% FBS for 7 additional days. Cells cultured under high glucose conditions began to form small clusters at appro- ximately day 7. After day 10, under the same conditions, the number and dimension of these clusters were markedly increased (Fig. 1). These formed clusters similar to islets of Langerhans as previously described by other investigators.
(9-12) The number of clusters obtained was 200±30/well.
2) Protein analysis of hUCB derived IPCs
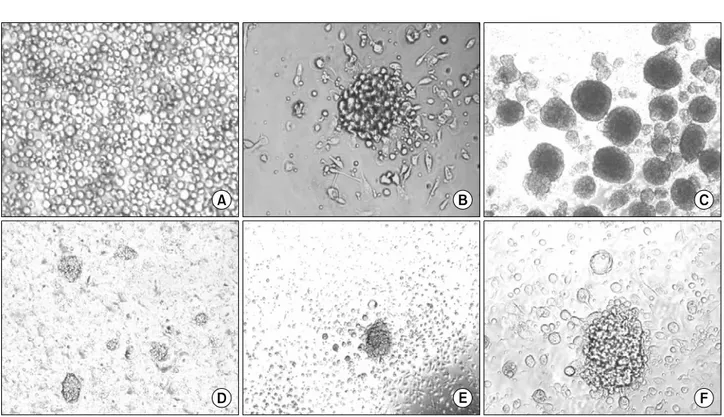
To investigate the expression of pancreatic hormones, im- munofluorescence analysis was performed for insulin in hUCB
derived clusters and human islets of normal pancreas. The human islets of normal pancreas were shown to strongly express insulin (Fig. 2). Uncultured hUCB were not stained with insulin (data not shown). The hUCB derived IPCs did express insulin. Insulin was expressed in cytoplasm.
A recent report demonstrated that insulin staining could be
artifactual, reflecting insulin uptake by apoptotic cells from
culture media containing high concentrations of insulin.(15-17)
The resulting controversy, which has been thoughtfully ad-
dressed by Kania et al,(18) has pushed the field to measure not
only insulin but also C-peptide, which is excised from
proinsulin, to prove that insulin has been synthesized. So,
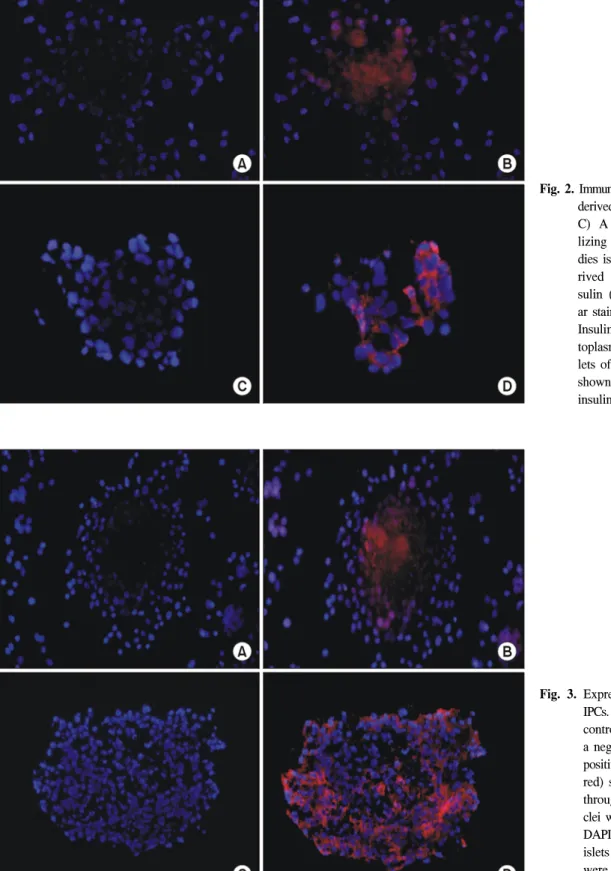
immunofluorescence was performed for C-peptide expression in
IPCs (Fig. 3). IPCs positive for C-peptide staining were ob-
served throughout the cluster. It showed a similar pattern of
staining from normal human islets as a positive control. The
C-peptide staining pattern of human islets from normal pan-
creas overexpressed insulin. These results suggest that hUCB
derived IPCs may possess the function of insulin secretion as
well as display morphology similar to islets.
Fig. 3. Expression of C-peptide in IPCs. (A, C) Matched isotype control antibodies served as a negative control. (B) IPCs positive for C-peptide (Cy-3, red) staining were observed throughout the cluster. Nu- clei were counterstained with DAPI (blue). (D) The human islets of normal pancreas were stained as a positive control (×200).
Fig. 2. Immunofluorescence of hUCB- derived cells with insulin. (A, C) A negative control uti- lizing isotype-mated antibo- dies is shown. (B) hUCB-de- rived IPCs stained with in- sulin (Cy-3, red) and nucle- ar staining with DAPI (blue).
Insulin was expressed in cy- toplasm. (D) The human is- lets of normal pancreas were shown to strongly express insulin (×200).
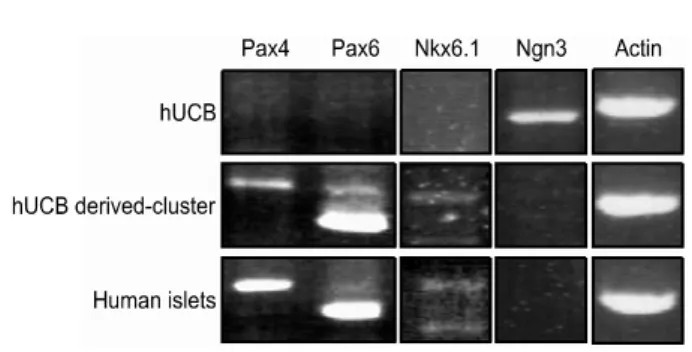
Fig. 4. Gene expression pattern by RT-PCR: The expression of Pax4, Pax6, and Nkx6.1were increased and Ngn3 was decreased in hUCB derived-cluster cultures.
3) RT-PCR (reverse tanscription-polymerase chain re- action)
To determine whether the islet-like clusters appearing in the hUCB derived cell cultures may have trans-differentiated into endocrine hormone-producing cells (especially IPCs), the ex- pression of transcription factors (Pax4, Pax6, Nkx6.1, and Ngn3) considered important for β-cell differentiation genes was examined by RT-PCR (Fig. 4). Ngn3 gene was decreased in hUCB derived-cluster cultures and human islets. The expression of Pax4, Pax6, and Nkx6.1were increased in in hUCB derived-cluster cultures and human islets.
DISCUSSION
The increasing prevalence of chronic human diseases, e.g., cardiovascular disease, diabetes and neuronal degenerative di- seases, presents a challenge to find more effective therapies.
Stem-cell-based therapy, including embryonic and adult stem cells, provides a rational treatment tool for regenerative me- dicine and has potential to revolutionize modern therapeutics.
Because of their high potential for self-renewal and pluripotent differentiation capability, ES cells have become a very active area of investigation. Ethical concerns, however, have limited their availability and practical usefulness. Leaving aside these ethical concerns, using in vitro fertilization (IVF) and altered nuclear transfer (ANT) to generate ES cells is made pro- blematic by the complexity of required technologies.(19) Adult stem cells are self-renewable and are found in many adult tissues. Recently, many researchers have been interested in adult stem cells because these cells have enough plasticity as well as there is no need to consider ethical problems. Hema- topoietic tissue includes extravascular BM, peripheral blood (PB) and UCB as sources of cells. Many reports on hema-
topoietic tissue-derived cells generating solid organ tissue- specific cells are based on BM- or PB-derived cells injected systemically or locally.(20) UCB cells have gained much interest as a stem cell source in the clinical transplant setting, in part because of their easy availability as a ‘cellular waste product'. When compared with adult BM-derived cells, the in
vivo engraftment of hUCB CD34+cells in a triple HLA- mismatched severe combined immunodeficient (SCID)-human bone model was markedly superior to adult BM-derived cells.(21) When using ex vivo-expanded BM- or CB-derived cells in the same model, CB-derived cells again showed superior levels of multilineage engraftment. Based on those hematopoietic engraftment observations, one may speculate that CB-derived cells express a higher potential to differentiate into or fuse with solid organ tissue-specific cells after systemic or local injection.
Recently, hUCB has been used as a source of stem cells to repopulate the hematopoietic system and other organs. Our data demonstrate that hUCB cells generate IPCs when cultured under DMSO and high-glucose conditions. These formed clusters similar to islets of Langerhans as previously described by other investigators. The number of clusters obtained was 200
±30/well. Glucagon like peptide 1 (GLP-1)/exendin-4 have in- cretin effects, enhancing insulin secretion; they also stimulate β-cell replication and neogenesis and have anti-apoptotic effects.(22) Betacellulin, a member of the epidermal growth factor (EGF) family, stimulates β-cell proliferation.(23) Both activin A, a member of the TGFβ family, and gastrin are thought to promote β-cell differentiation.(24) So, trials are being initiated with various combinations of gastrin, EGF and GLP-1 agonists, and no doubt other approaches to increase the genera- tion of IPCs from hUCB derived cells in vitro. To determine whether the islet-like clusters appearing in the hUCB derived cell cultures may have trans-differentiated into IPCs, the ex- pression of a number of transcription factors (Pax4, Pax6, Nkx6.1, Ngn3) considered important for β-cell differentiation was examined by RT-PCR. Strategies to differentiate progenitor cells into β-cells in vitro have been concerned as an alternative to increase β-cell availability prior to transplantation. It has recently been suggested that nestin-positive cells could be multipotential stem cells capable of expressing endocrine markers upon specific stimulation; however, this issue still remains controversial.
Our data represent that hUCB MNCs derived cells, when
cultured under serum-free medium containing DMSO and
followed by high-glucose condition, trans-differentiate into
IPCs which are endocrine cells capable of the production and
secretion of physiologically active insulin in vitro. It will be essential to understand what mechanisms are involved during the trans-differentiation process allowing hematopoietic cells to mature and differentiate into fully functional β-cell. Further- more, our data support that hUCB can be a potential source for the therapy of diabetes.
REFERENCES
1) Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC.
Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967;61:827-37.
2) Hakim NS. Pancreatic transplantation for patients with type I diabetes. Transplant Proc 2003;35:2801-2.
3) Moskalewski S. Isolation and culture of the islets of Langerhans of the guinea pig. Gen Comp Endocrinol 1965;44:342-53.
4) Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 1967;16:
35-9.
5) Weir GC, Bonner-Weir S. Scientific and political impediments to successful islet transplantation. Diabetes 1997;46:1247-56.
6) Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, War- nock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type I diabetes mellitus using a glucocorti- coid-free immunosuppressive regimen. N Engl J Med 2000;343:
230-8.
7) Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, Mckay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001;292:1389- 94.
8) Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK.
Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA 2002;99:16105-10.
9) Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA 2000;97:
7999-8004.
10) Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 2000;6:278-82.
11) Yang L, Li S, Hatch H, Ahrens K, Cornelivs JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem
cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci USA 2002;99:8078-83.
12) Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-dif- ferentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest 2004;84:607-17.
13) Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest 2003;111:
799-801.
14) Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Morphological and bio- chemical observations. Exp Cell Res 1975;94:70-8.
15) Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA.
Insulin staining of ES cell progeny from insulin uptake. Science 2003;299:363.
16) Hansson M, Tonning A, Frandsen U, Petri A, Rajagopal J, Englund MC, Heller RS, Hakansson J, Fleckner J, Skold HN, Melton D, Semb H, Serup P. Artifactual insulin release from differentiated embryonic stem cells. Diabetes 2004;53:2603-9.
17) Sipione S, Eshpeter A, Lyon JG, Korbutt GS, Bleackley RC.
Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia 2004;47:499-508.
18) Kania G, Blyszczuk P, Wobus AM. The generation of in- sulin-producing cells from embryonic stem cells-a discussion of controversial findings. Int J Dev Biol 2004;48:1061-4.
19) Melton DA, Daley GQ, Jennings CG. Altered nuclear transfer in stem-cell research-A flawed proposal. N Engl J Med 2004;351:2791-2.
20) Korbling M, Estrov Z. Adult stem cells for tissue repair: a new therapeutic concept? N Engl J Med 2003;349:570-82.
21) Rosler ES, Brandt JE, Chute J, Hoffman R. An in vivo competitive repopulation assay for various sources of human hematopoietic stem cells. Blood 2000;96:3414-21.
22) Li L, Yi Z, Seno M, Kojima I. Activin A and betacellulin:
effect on regeneration of pancreatic beta-cells in neonatal streptozotocin treated rats. Diabetes 2004;53:608-15.
23) Brubaker PL, Drucker DJ. Glucagons like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 2004;145:2653-9 (Minireview).
24) Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A.
Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet β-cells from pancreatic duct cells and an increase in functional β-cell mass. J Clin Endocrinol Metab 2005;90:3401-9.
Purpose: Here we showed that human umbilical cord blood (hUCB)-derived cells, when cultured under defined
conditions, generated insulin-producing cells (IPCs). Methods: hUCB mononuclear cells (MNCs) were cultured in serum-free low (5.5 mM glucose) DMEM at a cell density of 3×106/cm2 in the presence of 1% DMSO for 3 days followed by high (25 mM glucose) DMEM supplemented with 10% FBS for 7 additional days. They were plated in plastic six well plates on slide coverslips (22×22 mm2) coated with 0.006% type I collagen. Results:These IPCs formed clusters similar to islets of Langerhans. We confirmed these clusters were positive for insulin and C-peptide by immunohistochemistry. Conclusion: Our data demonstrated that in vitro hUCB-derived cells generated IPCs, which can be a potential source for the treatment of diabetes via a stem cell therapy approach. (J Korean Soc Transplant 2007;21:31-37)