Journal of the Pharmaceutical Society of Korea 21, 146-158 (1977)
The Crystal and Molecular Structure of
^-Cyclohexyl-A^ Co-Chlorobenzal) Imino Thiourea
C h u n g H o e K o o , H o j i n g K i m H o o n S u p K i m a n d C h o n g W h a n C h a n g
Department o f Chemistry, College o f N a tu ra l Sciences,Seoul N ational University, Seoul 151
(Received June 28, 1977)
Abstract—iVi-Cyclohexyl-iV2~Co-chlorobenzaO imino thiourea, C14H18
N 3SCI, crystallizes in C2/C, with a =19. 68, b=7.74, c=20. 42A, !8=
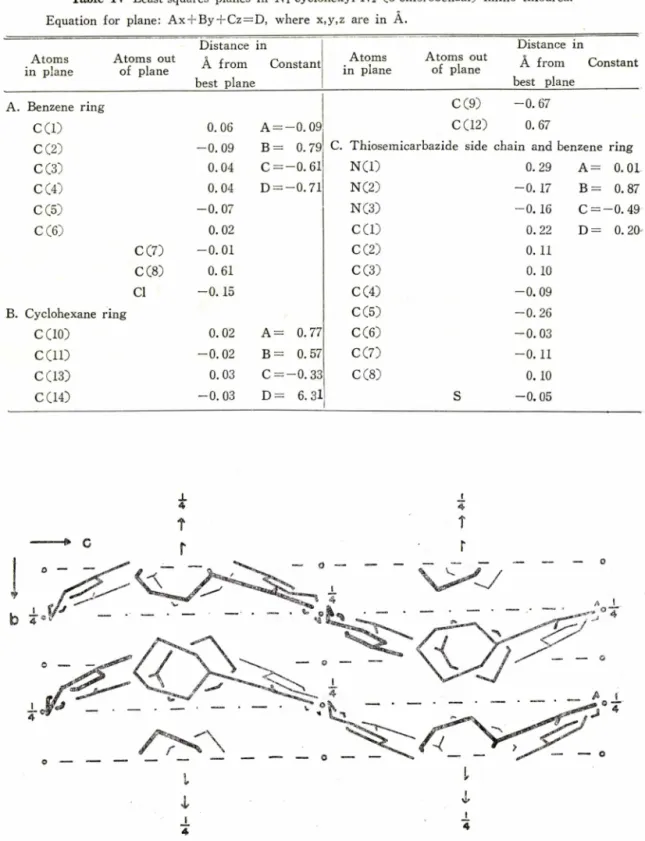
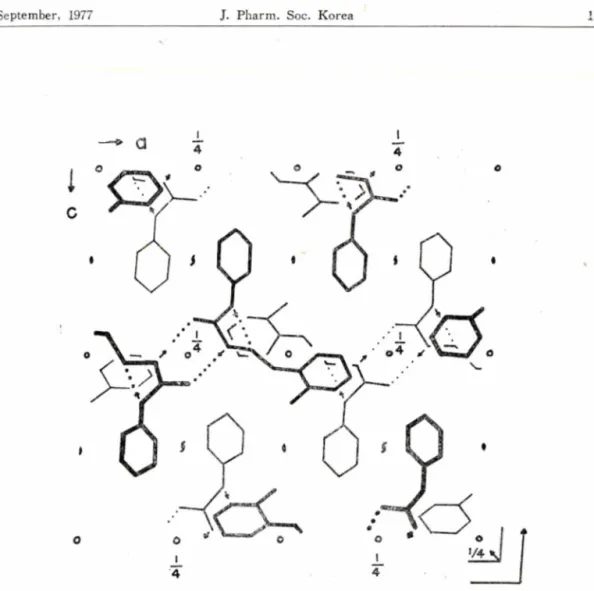
92. 8° and eight formula units in the unit cell. The structure was solved by the study of Patterson sections, calculated from three-dimensional film data, and was refined by block-diagonal least-squares methods to R = 0 .16 based on 1288 independent intensity data. The rest atoms of iVi-cyclohexyl-iV2~Co-cholorobenzal) imino thiourea molecule excluding cyclohexan ring and chlorine atoms approximately lie on a plane. A pair of molecules related by the symmetry centers are connected directly with the N-H S hydrogen bonds. Apart from the hydrogen bonding system the structure is held together by the van der Waals forces.
A large number of aromatic and heterocyclic thiosemicarbazone derivatives with antibacte
rial and antitumor activity were reported by French, et a l.1^ Their hypothesis was that a thiosemicarbazone which could function as a tridentate chelate capable of form ing octahedral complexes with metal ions would possess antibacterial and antitumor activity. M uch later M athew and Palenik4) showed, by precision X-ray diffraction studies, the octahedral form ula
tion to be correct for bis(isoquinoline-l-carboxaldehyde thiosemicarbazanato nick e l(II) mon- ohydrate. They found the two ligands tridentately bound in two orthogonal planes.
A comparison of the results of Palenik and coworker's55 structural studies on antitumor active 5-hydroxy-2-formylpyridine thiosemicarbazone sesquihydrate and inactive acetone thio
semicarbazone with the available structural data on other thiosemicarbazones suggested some generalizations regarding the electronic structures, complexing abilities and biological activities- of thiosemicarbazones. They suggested that planar mono-negative tridentate nature of thio
semicarbazones appears to be an essential feature for the activities.
Apparently a knowledge of conformation and bond lengths and angles which result from structure analysis could be much meaninigful for the study of biological activity. Therefore”
a crystal structure analysis of iVi-cyclohexyl-iV^Co-chlorobenzal) im ino thiourea was undertaken as part of a program devoted to explain the relationship between three dimensional structure of thiosemicarbazone and its biological activities.
E X P E R IM E N T A L
Suitable evenly developed single crystals were grown by slow evaporation from a solution of Ny A^-dimethyformamide at room temperature. Oscillation and Weissenberg photographs were taken with crystals mounted along the b and c axes respectively w ith C u K a radiation.
The unit cell dimensions were determined from the two zero-layer Weissenberg photographs, on which the diffraction lines of alum inium foil were superposed for calibration.
The density of crystal was measured by the floatation method in a m ixture of benzene and carbon tetrachloride and agrees well w ith calculated value.
Crystal data:
A^i-Cyclohexyl-A^Co-chlorobenzal) im ino thiourea, C14H18N3SCI. M W = 259. 8. monoclinic, 沒二 19.68土0 .05, 노二7. 74±0. 03,c = 2 0 . 4 2 ± 0 .05 入,/3=92. 8土0. 30,
V —1691 A 3, D m= 1 .2 6 , D x 二 1 .2 6 g cm-3, Z 二 8
Systematic absences (hkZ for h + k ~ 2 n-f-1, hOZ for 1—2 n+ 1 and h = 2 n + 1 and OkO for k —2 n + l)
were consistent w ith space groups Cc and C2/C. The centric space group was confirm ed by the successful solution and refinement of the structure.
For determination of the structure, intensity data were collected w ith C u K a radiation fo r layers hkZ, k = 0 to 6 and hkl9 1= 0 and 1, by m ounting the crystals about the b and c axes,
respectively with an equi-inclination Weissenberg goniometer, using the m ultiple film technique.
The relative intensities were estimated visually w ith aid of a set of graded intensities recor
ded for the same specimen. A total of 1288 independent reflections was observed. The inten
sities were corrected for the spot-shape, Lorentz and polarization factors. N o corrections either for absorption or extinction were made.
The intensities were then scaled to a common base by correlating various layers. A n overall scale factor 13.9 and overall temperature factor B = 2 . 45 A2 were computed by W ilso n's method6).
ST R U C T U R E D E T E R M IN A T IO N A N D R E F IN E M E N T
A three-dimensional sharpened Patterson synthesis was evaluated, and the prom inent peaks on Harker section and line were easily interpreted as Cl-Cl vectors. A structure factor calcu
lation w ith chlorine contribution alone gave an R index of 0.54. T he possible positions for the S atoms could be deduced from a chlorine-phased electron density map on (h0/) calculated w ith 128 reflections. The R index based on the chlorine and sulfur atoms was 0.49 for the 1044 reflections. A subsequent three-dimensional Fourier synthesis showed the nineteen peaks
which were consistent with a chemically reasonable model for the molecule. A t this stage the structure factor calculation for 19 atoms gave a discrepancy index R二0.35, with a uniform isotropic temperature factor, 2.45 人2.
Then the structure was refined isotropically using an IB M 1130 block-diagonal least-squares program by Shiono(1968)7). The quantity minimised was X]w(|F0| — |FC|)2. The weighting scheme proposed by Cruickshank(1965)8) was used throughout the refinement. The form of the function, w, was (a-f-1F01 +c | F 012) -1, where a = 2 | F min| =:4 0 .10 and c = 2 /| F max| = 0 .0 0 7 and the refinement was terminated where none of the parameter shifts exceeded one sixth of the corresponding estimated standard deviations. Final R index is 0.16 for all observed reflections.
The positional and thermal parameters for the non-hydrogen atoms together w ith their • estimated standard deviations are listed in Table I. A tom ic scattering factor values were taken from the International Table for X-ray crystallography9). The observed and calculated structure factors of the observed reflections are listed in Table II.
R E S U L T A N D D IS C U S S IO N
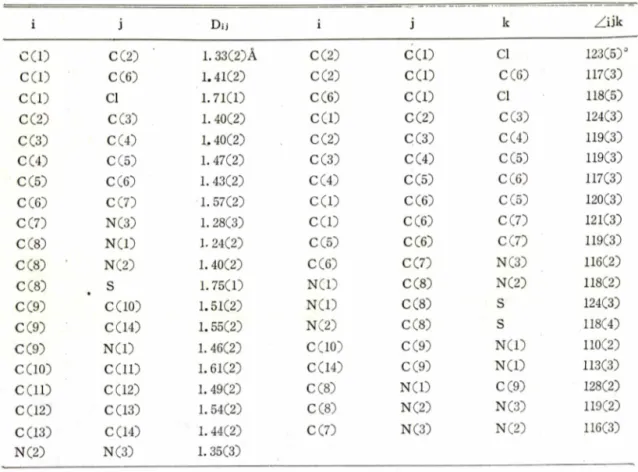
The bond lenths and angles are given in Table I I I and Fig. 1 where the numbering of the atoms is indicated.
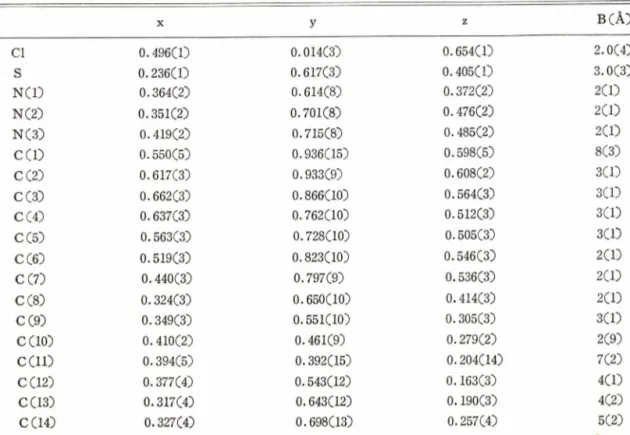
Table I -Final atomic coordinates and isotropic thermal parameters. The estimated standard deviations given in parentheses refer to the last decimal positions
X y z B (A )
Cl 0.496(1) 0.014(3) 0.654CO 2.0CO
s 0.236C1) 0.617C3) 0 .405CO 3.0(3)
NCD 0.364(2) 0.614(8) 0.372C2) 2CD
NC2) 0.351(2) 0.701C8) 0 .476C2) 2C1)
NC3) 0 .419C2) 0.715C® 0.485C2) 2C1)
CCO 0.550(5) 0.936(15) 0.598(5) 8(3)
c ⑵ 0.617(3) 0.933(9) 0.608(2) 3CD
CC3) 0.662(3) 0.866CL0) 0.564C3) 3CO
CC4) 0.637C® 0.762(10) 0.512(3) 3(1)
CC5) 0.563(3) 0.728(10) 0.505(3) 30D
CC6) 0.519C3) 0.823C10) 0.546C3) 2C1)
CC7) 0.440(3) 0.797(9) 0.536(3) 2(1)
C ⑧ 0.324(3) 0.650(10) 0.414(3) 2(1)
CC9) 0.349(3) 0.551(10) 0.305(3) 3CD
CC10) 0.410(2) 0.461(9) 0.279(2) 2(9)
c e i l ) 0.394(5) 0.392(15) 0.204(14) 7 ⑵
CC12) 0.377(4) 0.543(12) 0.163(3) 4CD
CC13) 0.317C4) 0.643C12) 0.190(3) 4(2)
CC14) 0.327(4) 0.698(13) 0.257C4) 5(2)
h=23 k = l h= 8 k = 0
123.14 104.04 141.42 118.67 208.44 191.28
81.64 70.63 21.43 14.48 41.91 33.05 17.05 11.99 34.61 26.39 9.72 6.18 h=10 k = 0 2 119.50 4 112.97 6 157.39 8 64.10 10 214.26 12 55.95 16 33.96 22 23.98
h = 0 k == 0 4 39.13 35.03 6 49.77 45.47
8 45.17 36.46
10 67. Z6 54.11 12 36.74 28.58 16 65.68 50.08 20 46.47 36.65
h=2 k == 0
h = 2 k = 2 0 68.8b 61.20 h=13 k = l
Table 11-Observed and calculated structure factors. Columns are: Index, Fobs,F cai
5
9
4
8
0
6
8
4
4
3
7
2
2
7
7
2
6
0 01-
7
1
8
7
0
717. 0
1
0
2
2
6
Cs
j 3
7.3.
1.
4.
6.
3.
4.
9.
1.
3.
0.
7.
7.
2.5.
7.
0.
4.
2
2
9
5
4
7
4
4
2
2
2
5
2
3
2
||
1
2
0
^ 2
0
1
1
k
2
0
9
3
5
2
5
0
1
4
2
9
9
8 4
9
4
0
4
6
5
1
9
0
0
1
8
4
2
3
6
4 10. 5. 4. 7.
=0 17. 55. 77. 75. 73. 41. 47. 35. 28. 31. 71. 32. 39. 32. h
1 2
2
3
3
9
3
1
4
7
6
7
6
1
2 1
8
4
3
7
7
9
3
9
1
6
9
1.
2.
4.
4.
0.
9.
4.
7.
7.9.
7.
3.
2
6
4
2
.
—
<
3 1
8
5
5
2
6 8
4
2
9
0 9. 6. 1. 8. 3. 1
2
1 5
0
6
9
1
3
8
0
8
5
3 0
1
1
9
4
1
1 6
1
4
8
2
1
7
1
4
6
3
3
5
8
9
5 1
8 J4. 5. 0. 4. 5. 4. 4. 3. 1. 2. 0. 6.
=1C V5
4. 6. 3. 3. 2 7
3
5
2
2
4
2
1
1
II1
2
3
1 1 h 8
9
0
1
2
3
4
6
8
9
0
1
1
6
0
4
0
1111111122112
4
2
6
5
7
3
8
0
2
2
0
1 4 8
5
2
5
7
0
3
6
7
8
5
1 2 6.
8. 9.
0. 7.
5.
2.
8. 7.1
7.
1. 3.
2 .1 4
2
2
2
1 1
II||
3
0
4
8
7
0
5
0
9
k
1 8
0
9
k
4 2
&
o 7 4.
001
00
9
3 o 4 5
1
8.
1.
5.
2.
9.
9.
7.
2.
1.
=11.
2.
3.
2.
=2 li5132c\Jll>—-m—
I ||
1
h
h 1 1 3 4 5 0 1 3 6
8
1
5
6 1
1 1
1
1
i—i
9
8
2
8
5 7
6
0
0
8 7. 0. 0. 6. 0. .—
l CvJ
1 3
9
7
1 2 4
7
3
2
3 1. 2. 7. 9. 5. 1
1
2
1 o
1
4
173 0
2
4
8
2
6
8
0
2 1
1
1
2
2 . -
i
9
6
3
0
4
1
7
2 6
8
7
2
0
9
7
5 2
. 43.
1
1
3
7
7 0
9
4
5
8
4
2
1
6
9
0
4 4
4
4
3 4. 6. 5. 0.
4- Ti 00 Cvj
3
3
8
8 8
2
7
2 4. 0. 4. 5. 5
2
4
2 0
2
4
7
h=5 k =
4
2
5
0
4
8
2
7
4
9
5
0
8
1 1
2
4
2
3
9
9
5
5
3
2
5
1
1 7
1
0
6
1
3
73. 2. 1. 0. 5. 1. 9. 6 0 0
1 0 1 4 5 33,
7
3 6
1 4'
—
_
1
1
II
0
)18
7
7
5
6
5
9
6
3
0
6
2 k. 1
0
7
5
3
4
2
8
9
2
7
2 MO
6 1
3
7. 2. 4. 4. 8. 2. 4. 8. 5. 9. 1. 2.
-7
6
8
6
2
4
4
2
4
8
2
7
1
5
2 11 1
h
7
0
8
2
5
9
7
8
2
6 9
6
9
1
0
1
1 2
5
3
7
8
0
5
3
3
3
5
1
3
5
7
6
2
8.
P.
7.
7.
4.
8.
2.
9.2.
6.
4.
7.
4.
2.
6.
1.
3.
1
2
2
4
4
1
2
4
1
1
2
1
1
1
1
1
11
6
2
0
6
8
2
1
4
4
4
7
1
3
9
2
5
4
k 4
1
5
6
8
9
7
3
1
5
5
6
0
5
1 9
7 90.
6 6. 1. 4. 9. 3. 7. 6. 7. 9. 9. 2. 4. 2. 4. 7.
=9
1
3
o :, 5
5
1
o :' 5
1
1
3
1
2
1 1
II
6
5
2
1
3
3
3
1
9
1
5
8
5
9
0
6
3
3
7 )07
)6
)6
17
)1
4
0
2
1
1
6
4
0
6
5
6
3
4
8
8
8
4
2
0
9
2
5
9
4
3
6.
3.
2.5.
9.
6.
0.
8.
3.
2.1
s
6
o o 4. - 7
1
3
0 0
2
1
~l
-—
1 4
2
3
2
6
4
1
7
3
5
3
1 4
5
8
1
5
6
6
1
1
8
k
4
2
0
8
7
3
9
5
7
5
0
1
6 179
LO
0 0 4
* Qo Ta T A4 - Tx
CN
J XI
LQCOlO
T A4 -
Cv
J 0 0 o
CTo
5 0 0
7« /*-•
Ti 6. 9.
3.
5.
1.
0. 4.
2.
2.
6.=
2.
0. 9.
0.
6. 3.
7.
9.
18.
1.
6. [43 2
9
5
3
5
7
2
3
5
3
1
II
2
6
2
3
2
7
4
2
8
4
6
4
1
1
1 1 11 2
0 2o 4 5 6
5 0
2
3
8
9
0
1
4
4 1
1
1 1 1
2
21
0
1 2
3
4
7
9
2
3
8
i--
1 1 1
* 2
3
4
5
6
7
8
190
12
14
15
17
22
14 45.22 C6.18 18 13.49 12.67
2
8
5
2
4
2
0
8
1 1 5
2
2
4
1
1
2 J 4
. 9 4
o
5 1
2 7
6
9
6 7. 3. 4. 2. 3. 3
TJ 6
2
2 o
2
4 8
10
II 1 1 1 1 II k
k
1 8
6
9
2 8
4
5
4 1
6
o
=1 6.
7.
2.
D.
1.
=2 II
2 1 2 2 1
||
h
h 2
8
0
2
4 1
2
1
6066
3
4
6
3 15. 18. 10. 11. 3
4
6
2 2
2
7
8 9. 2. 5. 6. 1
2
1
1 2
4
0
2 1
1
0 18.42 12.56 2 11.54 7.28
h=24 k=0 2 11.07 6.83 4 13.48 9.68
8
2
8
1
1
0
8
8
4
3
2
2
2 8
3
7
8
8
1
5
5
2
8
5
0 6 1 2 9 4 8 0
^ u
^ n _ 0
6
3
6
5
6
9
6
1
8
5
1
9
8
9
0
9
8
7
3
3
1
1
7
0
7
4
7
9
7
7
5
6
3
9 3. 9. 7. 6. 1. 8. 0. 6. 5. 2. 5. 0. 7. 7. 9. 8. 6. 5. 1. 7. 4. 5. 3. 2. 9. 6. 6. 4. 7. 9. 06.
3
6
8 I
5
8
6
8
2
o o 4
6
4
5
1
2
1
1
1
6
4
1
5
5
6
7
7
6
3
3
2
1
1 1
--
0
^ 1
2
1
1 k
9
7
8
6
3
0
2
3
9
3
2
8
2
3
k
6
7
9
8
7
2
2
1
7
3
6
6
1
3
4
7
6 '4
)9
)9
)9
6
0
0
0
8
5
3
8
6
5
1
3
2 1
1
2
7
2
1
4
9
0
2
2
1
0
3
1
2
9
4
0
^ 6 0 8 6
-1 0.
8.
4.
2.
8.
6.
4.
6.
0.5.
4.
1.
2.
1.
=3 9.
4.
4.
7.
1.
6.0.
5.
9.
5.
2.
6.
4.
9.
7.
1.
2.
2.
5..9.
소
II 5
7
6
8
1
4
4
7
5
6
1
2
2
2
||
0
4
8
4
4
7
5
8
8
3
4
3
1
1
1
1 1 1
lf i 2
1
1
1
1 4
5
6
7
8
9
1
2
3
4
5
6
7
8
9
^
^
TA r—I
r-H
i-HTi
Tx Tx
C\Ti
J C\
1
h
0 1 2 3
02
52
39
19
76
. 11
. 64
. 76
. 73
: f6
.89
. 00
.45
. 96
. 58 0 6 2. 4. 7. 7. 6o.
4. 5. 5. 0. 4o.
9.
>.8 L7. L2. t6.
3
4
4
4
5
||6
8
5
2
1 1
2
5
1 1
4 6
3
5
1
9
7
k
2
5
4
1 0
k
5
0
0 2 5
19
9
8
6
2
2 6
2
6
0
1 0
0
4
9
9
6
3
1
•
•
•
•
•
•
T x
•
•
•
•
• XI
• • 一
9 4
1
2
8
2
7
I-
2
8
4
7
0 -I 5
86
18
56
4
5
5
5
7
8
II
9
6
3
1
112
3
6
2
1
5 h
h 0
2
6
8 104
0
4
182 200
2
48
10
4
4
2
0
4
9
5
7 5
2
8
9
2
3
5
4 7. 2. 9. 2. 3. 5. 4. 1. o
8
3
4
3
3
4
2
1
o
II
1
II k
9
9
1
4
8
5
3
7
k 4
8
5
8
1
7
9
2
•
•
•
•
•
•
•
•
=
2
9
1
0
0
4
0
6
n
||
0
2
7
4 3
5
3
|| 1
h
1
1
h 0
2
4
0
4
6
0
4 1
1
1
2
2
9
6
9
7
8 5
6
6
5
2 6
2
9
0
1 5
0
7
9
1 7. 20. 6 8. 9. 9
6
2
2
3
0 6 8 2 6
i—HTA
g l 4 6
2
9
1
9
6
o
8
5
3
9
7
o
9
5
3
9
4
4
6
3
9
1
6
3
9
4
7
7
7
9
4
2
7
8
8
1
1
3
8
9 1 2
8 1
8
7
8
3 6 S 2
9
;8
)4
r 86
9 2
8
5
3
3
7
9
2
3
2
6
1
8
2
1
0
0
4
9
7
6
3
8
9
6
1
4
9
7
9
7
6
2
3
7
8
7
6
4
0
1
2
8
2
9
9
1
2
4
6
0
8
0
4
1
0
6
0
7
2 7 9 - H- 0. 5. 0. 7. 7. 4. 2. 4. 9. 2. 4. 1. 4. 3. 2. 1. 1. 7. 3. 0. 3. 9. 9. 5. 2. 9. 8. 8. 9. 9. 2. 3. 1. 9. 5. 5. 2. 9. 8. 5. 1. ecv.l gc. vi s.^ T
^ P i scv.i
^.
o.
^.
sc. o
2 8. 8. 1
4
6
2
4
3
8
9
2
2
3
5
1
1
2
7
1
4
1
3
4
6
3
6
5
4
4
4
3
2
4
3
6
2
7
5
1
1
2
2
1
2
1
8
4
3
6
5
6
3
1
1
2
1
2
1
||
6 7
1
=
=
II 1
k=
^ 1 9 0 9
9.
0
3
7
6
3
7
7
1
9
1
k
5
7
1
5
6
2
2
9
9
9
5
4
9
7
7
k
6
9
6
1
8
0
2
7
3
4
7
3
k
5
5
2
4
9
8
7
4
9
4 여 P 2 구 6
9
1 8 1 2 9 4
4
0
2
9
8
1
3
6
9
5
05
1 8 4 1
5
1
7
1
4
6
6
6
4
1 1
3 6 0 5 3 1 7 7 9 1 7 9
9 4 9 4 2 1 8 9 5 7 9 1 5 6 1
o
4 3 2.
>8. t8. 4. 5. 3. 8. 0. l.
9. 5. 9. 5. 5. 9. 5.
=4
6. 0. 7. 6. 7. 5. 6. 9. 5. 7. 2. 6. 8. 0. 3.
^6
6. 9. 3. 2. 6. 40. 6 5. 4. 1. 9. 5.
=8o d oo. d ga. J so. d
^.
e. LS.
^.
l^
^s. LZ.
^
=
r6. 56. 1
2
4
6
2
4
3
0
1
2
3
3
6
1
1
||
8
1
3
2
2
4
5
0 0.
7
5
5
4
5
4
-11
3
3
7
3
9
5
1
2
3
2
1
=
3
0
4
2
6
5
7
2
1
1
2
1
2
1
1 II 7 8
Ih
Ih - -
Tw
1
2
3
4
5
6
7
8
9
10
12
13
14
18
20
240
1
2
3
4
5
6
7
8
9
10
11
127
0
0
1
3
4
5
6
7
8
9
5
7
9
0
1
3
4
5
6
7
8
10
11
14
15
16
17
21o
1