170
FOREST SOCIETY
Depletion of Phosphorus in Mountain Soil and Growth Stimulation of
Panax ginsengby Phosphorus Enrichment
Yong-Eui Choi*, Myong-Jong Yi, Kyung-Ha You, Kee-Hwa Bae, Jung-Yeon Han and Jae-Seon Yi
Division of Forest Resources, College of Forest and Environmental Sciences, Kangwon National University, Chunchon 200-701, Korea
Abstract :There are remarkable differences in growth and morphological characters of roots between mountain and field cultivated Panax ginseng. Growth of root in mountain cultivated ginseng was much slower than that of field cultivated ginseng. However, the factor affecting the retarded growth in mountain ginseng was not known. Soil analysis revealed that phosphorus (P) content of mountain soil was exceptionally low at least ten-fold lower compared to that of field soil. Thus, we suggest that low availability of P in mountain soil may be one of the limiting factors for growth of ginseng in mountain soil environment. We had monitored the growth of ginseng plants after one and three years of phosphate fertilizer application. Three kinds of phosphate fertilizers: fused magnesium phosphate, fused superphosphate, and single superphosphate were applied to mountain soil. Application of phosphate fertilizers increased the fresh-, dry weight, and diameter of ginseng roots and resulted in increased P accumulation in roots. These results demonstrate that slow growth of ginseng in mountain soil environment might be attributed to the low P content in mountain soil. Thus, analysis of P amount in mountain soil will be a good indicator for the selection of suitable site the ginseng cultivation in forest.
Key words : Panax ginseng, growth analysis, phosphorus, root growth
Introduction
Ginseng (Panax ginseng C. A. Meyer) is a perennial herbaceous species, belonging to the family Araliaceae.
This species has long been used as an important medic- inal plants. Roots of ginseng, one of the most famous and expensive crude drugs, have been commonly used to promote life quality (Ellis and Reddy, 2002; Coleman et al., 2003). Immune system modulation, anti-stress activ- ity, anti-cancer, and anti-diabetic activities are the most notable features of ginseng in laboratory and clinical tri- als (Vogler et al., 1999; Shibata, 2001).
Recently, artificial cultivation of ginseng in forest has been rapidly increased in Korea. Ginseng roots culti- vated in forest sold by much higher price than field cul- tivated ginseng because they are free from agrochemicals and considered as similar medicinal values to highly pre- cious wild ginseng. In American ginseng (Panax quin- quefolius), wild- and forest cultivated ginseng roots are exported to abroad, exporting annually an average of 60 metric tons from the United States (132,300 pounds)
(Robbins, 1998).
Now it is accepted that mountain cultivated Korean ginseng will be one of the best non-timber forest prod- ucts. Despite the high demand of mountain cultivated ginseng roots, production of roots was confronted with serious problem because growth of roots is extremely slow in mountain forest environment (Choi et al., 2007).
Fresh weight of ginseng roots cultivated in agricultural field was reached to about 75 gram after 4 years of cul- tivation. Whereas, fresh weight of roots in mountain cul- tivated ginseng was around 5 g even after 10 to 15 years of cultivation (Choi et al., 2007). The low productivity of roots owing to the slow growth of ginseng plant in mountain is major limitation to the consumer. To support the growth of ginseng, farmers tend to transfer ginseng plants to fresh soil site at about three years intervals.
However, it is completely unknown why P. ginseng grows very slowly in mountain environments and why transferring of ginseng to new soil site stimulates the growth.
In Korea, available P content in mountain soil is around 10 mg/kg (Lee, 2008). Whereas, available P con- tent in the cultivated fields for agricultural crop is around several hundred mg/kg (Kim et al., 1995). Recently, Choi
*Corresponding author E-mail: yechoi@kangwon.ac.kr
et al., (2007) reported that mountain soil showed extremely low P content, at least 10-fold lower than that of field soil, whereas N and organic matter were rather richer in mountain soil than those of field soils. It was reported that P was a limiting factor for tree growth in natural environments (Sheriff et al., 1986; Topa and Cheeseman, 1992). Growth stimulation of plants in response to enhanced P availability was reported for many tree spe- cies either in the field or under artificial growth condi- tions (Chang, 2003; Sheriff et al., 1986; Topa and Cheeseman, 1992). Although the importance of P avail- ability in soil is extensively reported, there was no report on the availability of P for ginseng cultivation in forest.
The objective of this research was to investigate the role of P in the growth of ginseng plants in forest soil.
Three types of P fertilizers: fused magnesium phosphate (FMP), fused superphosphate (FSP), and single super- phosphate (SSP) were applied to mountain soil. We exam- ined the growth of ginseng plants and P uptake in roots after P fertilizer application.
Materials and Methods 1. P fertilizer application
Dehisced seeds of Panax ginseng were sown on the surface of mountain in Kangwon National University Research Forest situated at Hongcheon-gun. Seeds were evenly spaced in 10-cm distance. Before germination of seeds in spring, three types of P fertilizers (Kyoungki Chem- icalCo, products): fused magnesium phosphate [CaO·8MgO, P2O5·nSiO2], fused superphosphate [CaH4(PO4) + (3MgO, CaO, P4O5, 3CaSiO4)], and single superphosphate [CaH4
(PO4)2 CaHPO4H3PO4CaSO4-2H2O] were applied to moun- tain soil. Treated amount of fertilizers was about 100 g of fertilizer per 1 m2.
Plants were harvested after 1, 2, and 3 years of cul- tivation. After harvesting, the lengths, diameters, fresh and dry weights of the roots were recorded. At least 7 individual roots per sample were analyzed. Root length and diameter were measured using Vernier caliper. Bio- masses of driedshoots and roots were measured on a microbalance (Sartorius,Edgewood, New York, USA).
2. Mineral element accumulation in roots by ICP-AES analysis
To analyze the mineral accumulation in roots, roots were dried at 60°C for 24 hrs. Dried root samples were ground and sieved through a 200 mesh (about 0.074 mm pore size) nylon sieve for chemical analysis. Powdered sediment splits of 0.2 g were digested with 1 mL 5 N HNO3 for 24 hrs in a tightly closed Teflon vessel, and then dried on a hot plate at less than 150ºC. Afterwards, the dried extracts were ashed in a mufflefurnace (Ther-
molyne 48000, Dubuque, Iowa, USA) at 500°Cfor 5 hrs, and extracted with 5 N (10 mL) HNO3. After centrifuga- tion and filtration through membrane filter (0.2 μm cel- lulose acetate membrane filter, Millipore), major elements were analyzed with ICP-AES (Optima 4300 DV, Perkin Elmer Co.) in the Korea Basic Science Institute.
3. Physicochemical soil analysis
To check the amendment of P in mountain soils, soil samples were collected from either fertilizer treated- and non-treated soil three years after fertilizer application.
Prior to our physical and chemical analyses, all samples were air-dried at room temperature and passed through a 2-mm sieve. Soil pH was measured with an Orion 720+ pH meter (Orion Research, USA) at a 1:5 ratio with dis- tilled water for 1 hrs.
Soil organic matter was determined according to the Tyurin method (Bel'chikova, 1954). Total N was ana- lyzed on a micro Kjeldahl apparatus (B324; Buchi, Swit- zerland) following wet digestion in concentrated H2SO4
on a block digester (K438; Buchi) (Jackson, 1962). Avail- able P (P2O5) was measured with a UV-spectrophotom- eter (UV-2501PC; Shimadzu, Japan) via the Lancaster method (Cox, 2001), in which the extracting solution is based on the reaction with ammonium molybdate. Ex- changeable base cations were extracted with 1N ammo- nium acetate (pH 7.0) and then measured with a pH meter (F-53; Horiba, Japan). Exchangeable K+ and Na+ were determined by Flame photometry (PFP7; Jenway, UK), while Ca2+ and Mg2+ levels were obtained by Atomic Absorption spectrophotometry (280FS; Varian, USA), using 1N ammonium acetate as the extracting solution.
Results and Discussion
1. Slow growth of mountain cultivated ginseng (MCG) roots and low P level in mountain soil
In MCG roots, roots growth was much slower than those of field cultivated ginseng (FCG) roots (Figure 1).
Fresh weight of 5 year-old MCG roots was less than that of 2 year-old FCG roots. The mineral properties between mountain and field soils were compared (Table 1). The amount of P (P2O5) in mountain soil was 14.7-time lower than that of field soil (Table 1). Contrary to P, total N and other cations were not highly different between field and mountain soils.
Lee (2008) reported that mean of available P content in mountain soil is 8.83 mg/kg in Gyeongsang province in Korea (Lee, 2008), and he interpreted that P is under the state of deficiency in mountain soil. Contrary to mountain soil, available P content in the agricultural fields for ginseng cultivation is around several hundred mg/kg (Kim et al., 1995). Choi et al. (2007) compared
the soils sampled from the root zone (rhizosphere) and from the non-root zone (20 cm away from the roots) of ginseng in mountain. Regardless the root age, available P level was remarkably decreased in the root zone. In general, the desirable amount of P for the growth of plants is about 60 mg/kg. In American ginseng (P. quin-
quefolius), appropriate concentration of available P for cultivation is 150-200 mg/kg (Li and Wallis, 1994). Taken together, among the other various environmental factors affecting the growth of ginseng, the exceptionally low amount of P in mountain soil could be one of major fac- tors for slow growth of mountain ginseng.
The low P in mountain soil is mainly caused from the soil erosion (Sharpley et al., 1992; Catt et al., 1998). P is carried in runoff water from mountain into streams,
wetlands, and lakes (Sharpley et al., 1992; Catt et al., 1998). P deficiency is manifested by a reduction in leaf size (Constant and Sheldrick, 1991; Cromer et al., 1993;
Rodríguez et al., 1998), and low photosynthesis of leaves (Rao and Terry, 1989; Kirschbaum and Tompkins, 1990;
Jacob and Lawlor, 1991).
2. Enhanced root growth by P fertilizer treatment SSP is a fast release phosphate fertilizer owing to high proportion of water-soluble phosphate (17% available phosphate and 13% water soluble phosphate). FMP is a slow-release phosphate fertilizer (20% citric acid soluble phosphate). Plant can utilize P in FMP after conversion into soluble form by the organic acid (citric acid) released from soil microorganism or from roots exudates, indi- cating not effective for the early seedling growth. FSP is a mixture of FMP and SSP (20% citric acid soluble phos- phate and 8% water soluble phosphate). SSP is manu- factured by treating phosphate rock with sulfuric acid to increase the solubility of the P it contains. Sulfuric acid converts the phosphate rock to calcium dihydrogen phosphate Ca (H2PO4)2, a water-soluble form that plants are able to utilize. FMP, naturally occurring phosphate rock contains a high proportion of calcium phosphate Ca(PO4)2 which is normally not water-soluble to be used as a fertilizer.
To demonstrate the influence of P on the growth of ginseng plants in mountain, 3 kinds of P fertilizers;
FMP, FSP, and SSP were applied to mountain soil. After 5 months of fertilizer applying, growth of plants was analyzed. As seen in Figure 2, both fresh- and dry weight of roots were remarkably increased after P fer- tilizer treatment. The diameter of roots was conspicuously thicker than that of non-treated soil (Figures. 2C, 3).
Growth of roots was different by the kinds of fertilizers applied. Among the treated fertilizers, SSP and FSP treatments were resulted in better growth than FMP treatment (Figure 2). We suggest that better growth of ginseng plants by SSP or FSP treatments is due to the amount of available P in P fertilizers because available P is different among fertilizers. It was known that plant requires adequate P from the early stage of seedling growth for optimum crop production (Grant et al., 2001).
Figure 1. Comparison of root growth in P. ginseng grown in mountain forest soil and farm field soil. A, Fresh weight increase in P. ginseng roots grown in mountain forest soil and ginseng farm field. B-C, Ten years old roots grown in mountain forest. D, Five years old roots grown in ginseng farm field. Bar in B, C = 12 mm. Bar in C = 17 mm.
Table 1. Comparison of physcochemical properties of soil in non-rhizosphere and rhizosphere of ginseng plants.
Soil pH Organic
matters
(g/kg) Total N
(g/kg) P (P2O5)
(mg/kg) Exchangeable cations (cmol+/Kg)
K+ Na+ Ca2+ Mg2+
Mountain 5.03 56.1±1.21 3.66±0.24 22.83±4.34 0.48±0.06 0.07±0.01 2.72±0.2 0.81±0.07 Field 5.63 34.9±4.16 1.38±0.27 335.5±29.68 0.50±0.08 0.30±0.05 2.65±0.17 0.58±0.07 MCG soils were sampled from Research Forest of Kangwon National University situated in Gangwon province.
Field soils were sampled from the ginseng farm field situated in Hongcheon-gun, Gangwon province.
Rhizosphere soils were sampled from root zone of ginseng plants.
After three years of phosphate fertilizer treatment, every growth values such as fresh-, dry weight, and diameter of roots were also remarkably higher in fertilizer treated soil than non-treated soil (Figure 4). Contrary to the data for 5 month-old plant, both fresh- and dry weight of roots were highest in FMP soil after three years of cul- tivation. The differences in the growth of roots between 5 month-old and three year-old plants might be resulted from the rate of chemical composition in fertilizer. In SSP treated soil, pH value was lower than those of non- treated soil (Table 2). Low soil pH in SSP treated soil could affect the growth of ginseng plants because soil pH is the most important soil environmental factor for the growth of plants (Jönsson et al., 2003). The growth stimulation of ginseng plants in FMP treated soil indi- cates that ginseng roots and microorganism near root zone can decompose the phosphate rock (FMP) into sol-
uble phosphate.
Yellowing of leaves was noticed in plants grown in fertilizer treated soil (Figure 5B), and this was more con- spicuous in SSP treated soil. Whereas, in control plants, leaves of plants were all green (Figure 5A). It was reported that P fertilization often resulted in leaf chlo- rosis and calcium deficiency of tomato in green house soil (Giuffré et al., 2004). Fertilizer P may have been occluded in the Fe oxides, particularly in the less crys- talline forms, which hampers the Fe uptake into plants (Ruiz et al., 1997). Thus, amount of fertilizer should be carefully considered to prohibit over treatment dose for ginseng cultivation in mountain.
3. Mineral uptake in roots
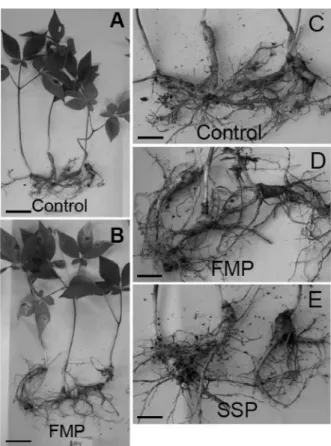
Mineral uptake in roots was observed by ICP-ACS analysis. Because 3 kinds of fertilizers contain different amounts of S, Mg, and Ca, and the accumulation of min- erals in roots was corresponded with the mineral com- positions of fertilizers (Figure 6). In all 3 kinds of phosphate fertilizer treatment, ginseng roots contained higher amount of P compared to non-treated roots (Fig- ure 6). FMP comprise about 12% of magnesium (Mg), Figure 2. Growth of ginseng roots grown for 5 months
under mountain forest after phosphate fertilizer application.
A, Fresh weight of roots; B, Dry weight of Roots; C, Diameter of root main bodies. FSP is fused superphosphate.
FMP is fused magnesium phosphate. SSP is single superphosphate.
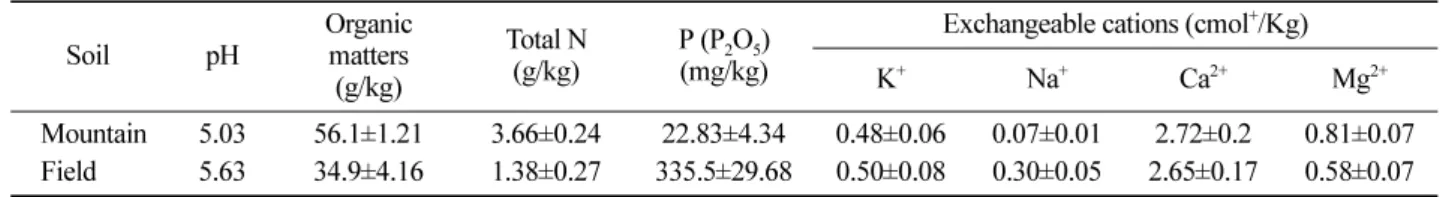
Figure 3. Photographs of ginseng plants grown for 5 month under mountain forest after phosphate fertilizer application. A, Roots of ginseng grown in control soil and SSP applied soil; B, Ginseng plants grown in control soil and SSP applied soil; C, Ginseng plants grown in FSP applied soil; D, Ginseng plants grown in FMP applied soil.
thus the roots grown in FMP soil contained highest amount of Mg compared to others (Figure 6D). SSP comprises about 8% of sulfur (S), thus the root grown in SSP soil contained highest amount of S compared to others (Figure 6E). SSP contained 29% of calcium (Ca), FSP 27%, and FMP 20%. The difference of Ca content
in fertilizers simultaneously affected the Ca uptake in roots (Figure 6B). These results indicate that absorption of minerals into ginseng plants was directly affected by chemical properties of P fertilizers, and high P uptake in root by P fertilizer treatment resulted in the stimulation of root growth.
4. Physicochemical soil analysis
Changes of mineral elements in soil were analyzed after 3 years of phosphate fertilizer treatment. It was found that P content in soil was enriched remarkably by phosphate fertilizer application (Table 2). The P content Table 2. Physcochemical properties of mountain soil after three years of phosphate applying.
sampleSoil pH Total N
(mg/kg) P (P2O5)
(mg/kg) Exchangeable cations (cmol+/kg)
K+ Na+ Ca2+ Mg2+
Control 5.03 3.66±0.24 22.83±4.34 0.48±0.06 0.07±0.01 2.72±0.2 0.81±0.07
SSP 4.88 2.75±0.56 409.34±20.78 0.27±0.05 0.19±0.04 2.86±0.37 0.23±0.05
FSP 5.61 3.50±0.37 424.49±19.24 0.65±0.06 0.29±0.03 6.13±0.69 1.36±0.19
FMP 5.26 2.85±0.55 304.83±23.57 0.25±0.03 0.08±0.01 4.72±0.72 3.60±0.45
Soils were sampled at 20 cm apart (non-rhizosphere) from ginseng plants.
MCG soils were sampled from Research Forest of Kangwon National University situated in Hongcheong-gun, Gangwon province.
Figure 4. Growth of ginseng roots grown in mountain forest for 3 years after phosphate fertilizer application. A, Fresh weight of roots; B, Dry weight of Roots; C, Diameter of root main bodies.
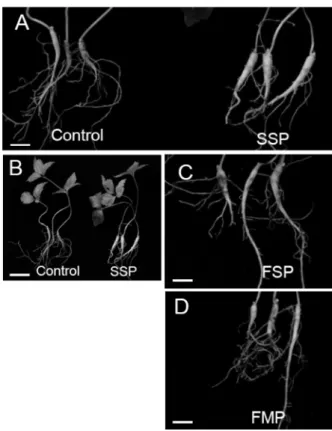
Figure 5. Photographs of ginseng plants grown for three years under mountain forest after phosphate fertilizer application. A-B, Ginseng plants grown in non-treated soil (A) and FMP applied soil (B); C-D, Roots of ginseng grown in non-treated soil (C), FMP applied soil (D), and SSP applied soil (E).
in mountain soil after phosphate fertilizer application was similar level to the soil sampled from agricultural ginseng farm field (Kim et al., 1995). Moreover, Na, Ca, and Mg were enriched in soil after fertilized treatment but K was unaffected (Table 2).
There is no report on the effect of soil fertilizer in the culture of ginseng in mountain forest. In P. quinquefo- lius, it was noted that the application of lime containing rich Ca in forest soil had a positive effect on growth of P. quinquefolius (Nadeau et al., 2003). They concluded that the positive growth by lime application could be related to higher calcium concentration and/or lower alu- minum toxicity. There are contradictory evidences on the effect of P fertilizer application for the growth of Amer- ican ginseng (P. quinquefolium L.). Konsler and Shelton (1990) reported that soil applications of limestone and bone powder (rich in Ca and P, respectively) before seeding of American ginseng affected root weight gain during the first 4 years of growth. Whereas, Li and Wal- lis (1994) reported that root length, diameter, fresh weight of roots, and total length of leaves were not
affected by phosphate levels in agricultural field exper- iment although higher phosphate levels increased seed emergence and reduced seedling mortality, indicating that root growth is not affected by phosphate fertilizer application in P rich soil. It has suggested that the annual N requirements of ginseng are quite small and that increasing the annual N application rate resulted in a decline in root yield in P. ginseng (Jinfang et al., 1997).
In conclusion, our results demonstrated that P content of the mountain soil may be critical for the root growth of ginseng. Phosphate fertilizer treatment is highly effec- tive for growth of ginseng plants in mountain environ- ment. Thus soil test, in particular for P, before cultivation of ginseng in mountain will be essential for recognizing of suitable cultivation site in mountain.
Acknowledgements
This study was supported by Korea Forest Service research fund.
Figure 6. Mineral contents in roots of ginseng grown for three years under mountain forest after phosphate fertilizer application. A, P content; B, Ca content; C, K content; D, Mg content; E, S content; F, Mn content in roots.
Literature Cited
1. Bel'chikova, N.P. 1954. Determination of soil humus content by Tyurin's method. In:Agrochemical Methods of Soil Analysis. Ed 2. Russian Academy of Science, Moscow, pp 35-42.
2. Catt, J.A., Chambers, B.J., Farina, R., Harris, G.L., Hodgkinson, R., Howse, K.R. and Quinton, J.N. 1998.
Phosphorus losses from arable land in England. Soil Use Manage. 14: 168-174.
3. Chang, S.X. 2003. Seedling sweetgum (Liquidambar styraciflua L.) halfsib family response to N and P fer- tilization: growth, leaf area, net photosynthesis and nutrient uptake. For. Ecol. Manage. 173: 281-291.
4. Choi, Y.E., Kim, Y.S., Yi, M.J., Park, W.G., Yi, J.S., Chun, S.R., Han, S.S. and Lee, S.J. 2007. Physiological and chemical characteristics of field- and mountain-cul- tivated ginseng roots. J. Plant Biol. 50: 198-205.
5. Coleman, C.I., Hebert, J.H. and Reddy, P. 2003. The effects of Panax ginseng on quality of life. J. Clin.
Pharm. Ther. 28: 5-15.
6. Constant, K.M. and Sheldrick, W.F. 1991. An outlook for fertilizer demand, supply, and trade, 1988/89–1993/
94, World Bank Technical Paper No. 137. World Bank, Washington, DC.
7. Cox, M.S. 2001. The Lancaster soil test method as an alternative to the Mehlich 3 soil test method. Soil Sci.
166: 484-489.
8. Cromer, R.N., Kriedemann, P.E., Sands, P.J. and Stew- art, L.G. 1993. Leaf growth and photosynthetic response to nitrogen and phosphorus in seedling trees of Gamelia arborea. Aust. J. Plant Physiol. 20: 83-98.
9. Ellis, J.M. and Reddy, P. 2002. Effects of Panax gin- seng on quality of life. Ann. Pharmacother. 36: 375- 10. Giuffré, L., Alconada, M., Pascale, C. and Ratto, S.379.
2004. Environmental impact of phosphorus overfertil- ization in tomato greenhouse production. J. Appl. Hor- ticult. 6: 58-61.
11. Grant, C.A., Flaten, D.N., Tomasiewicz, D.J. and Shep- pard, S.C. 2001. The importance of early season P nutrition. Can. J. Plant Sci. 81: 211-224.
12. Jackson, M.L. 1962. Soil Chemical Analysis. Consta- ble, London.
13. Jacob, J. and Lawlor, D.W. 1991. Stomatal and meso- phyll limitations of photosynthesis in phosphate defi- cient sunflower, maize and wheat plants. J. Exp. Bot.
42: 1003-1011.
14. Jinfang, G., Longnan, J., Shuwen, Z., Lanzhen, Z. and Shuren, Z. 1997. A phenomenon of nitrogen excess in Panax ginseng. - a preliminary study in the cause of nitro- gen toxicity to ginseng. Acta Pedologica Sinica 34:
206-211.
15. Jönsson, U., Rosengren, U., Thelin, G. and Nihlgård, B.
2003. Acidification-induced chemical changes in conif- erous forest soils in southern Sweden 1988-1999. Envi- ron. Poll. 123: 75-83.
16. Kim, D.C., Chang, S.M. and Choi, J. 1995. Effect of the chemical properties of field soils on the contents of sugars and saponin in ginseng roots. Agr. Chem. Bio- tech. 38: 72-77.
17. Kirschbaum, M.U.F. and Tompkins, D. 1990. Photo- synthetic responses to phosphorus nutrition in Eucalyp- tus grandis seedlings. Aust. J. Plant Physiol. 17: 527- 18. Konsler, T. and Shelton, J.E. 1990. Lime and phospho-535.
rus effects on American ginseng: 1. Growth, soil fer- tility, and root tissue nutrient status response. J. Amer.
Soc. Horti. Sci. 115: 570-574.
19. Lee, C.K. 2008. Studies on the relation between acid deposition and soil chemical properties in forest areas.
Kor. J. Env. Eco. 22: 260-267.
20. Li, T.S.L. and Wallis, M.O. 1994. Effect of soil phos- phorus levels on seed emergence, seeding mortality and plant and root development of American ginseng. J.
Ginseng Res. 18: 134 -136.
21. Nadeau, I., Simard, R.R. and Olivier, A. 2003. The impact of lime and organic fertilization on the growth of wild-simulated American ginseng. Can. J. Plant Sci.
83: 603-609.
22. Rao, I.M. and Terry, N. 1989. Leaf phosphate status and photosynthesis in vivo in sugar beet: I. Changes in growth, photosynthesis and Calvin cycle enzymes.
Plant Physiol. 90: 814-819.
23. Robbins, C.S. 1998. American ginseng: the root of North America’s medicinal herb trade. TRAFFIC North America Report, Number B347, Washington, D.C., 24. Robbins, C.S. 2000. Comparative analysis of manage-USA.
ment regimes and medicinal plant trade monitoring mechanisms for American ginseng and goldenseal.
Conserv. Biol. 14: 1422-1434.
25. Rodríguez, D., Zubillaga, M.M., Ploschuk, E.L., Keltjens, W.G., Goudriaan, J. and Lavado, R.S. 1998. Leaf area expansion and assimilate production in sunflower (Helianthus annuus L.) growing under low phosphorus conditions. Plant Soil 202: 133-147.
26. Ruiz, J.M., Delgado, A. and Torrent, J. 1997. Iron-related phosphorus in overfertilized European soils. J. Environ.
Qual. 26:1548-1554.
27. Sharpley, A.N., Smith, S.J., Jones, O.R., Berg, W.A.
and Coleman, G.A. 1992. The transport of bioavailable phosphorus in agricultural runoff. J. Environ. Qual. 21:
30-35.
28. Sheriff, D.W., Nambiar, E.K.S. and Fife, D.N. 1986.
Relationships between nutrient status, carbon assimila- tion and water use efficiency in Pinus radiata needles.
Tree Physiol 2: 73-88.
29. Shibata, S. 2001. Chemistry and cancer preventing activity of ginseng saponins and some related terpenoid compounds. J. Kor. Med. Sci. 16: S28-37.
30. Topa, M.A. and Cheeseman, J.M. 1992. Carbon and phosphorus partitioning in Pinus serotina seedlings growing under hypoxic and low-phosphorus conditions.
Tree Physiol. 10: 195-207.
31. Vogler, B.K., Pittler, M.H. and Ernst, E. 1999. The effi- cacy of ginseng: A systematic review of randomized clinical trials. Eur. J. Clin. Pharmacol. 55: 567-575.
(Received January 19, 2009; Accepted March 4, 2009)