Anti-allergic Effect of Seungmagalgeun-tang through Suppression of NF-${\kappa}B$ and p38 Mitogen-Activated Protein Kinase Activation in the RBL-2H3 Cells
전체 글
수치


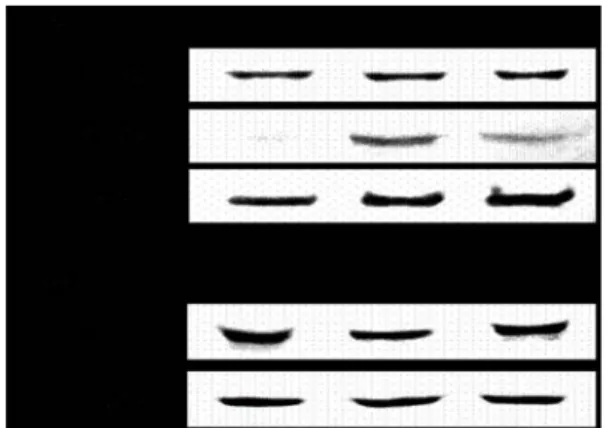

관련 문서
Just as we achieved good control with a joint-based controller that was based on a linearizing and decoupling model of the arm, we can do the same for the Cartesian case.
In this study, we tested whether a potent AMP-activated protein kinase (AMPK) activator, nectandrin B inhibits VSMC proliferation and neointiima formation and tried to
In the region of Auerbach’s plexus (AP) of small intestine, the staining intensity of the ICC-IR cells was reduced in the HFD group compared to the control group.. The numbers
Conclusions: Thus, 25-HC induced odontoclast differentiation through the odontoclastogenesis factor-mediated activation of NF-κB and upregulated inflammatory
Often found connected to other molecules on the outsides of cells --- cellular recognition, cell signaling, cell
Background : Janus tyrosine kinase 2 (JAK2) V617F mutation has been described in a high proportion of patients with Philadelphia
Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia and myeloid metaplasia with myelofibrosis.. A gain-of-function
Glutamate excitotoxicity induced by excessive activation of NMDA receptor causes various damage to cells, which leads to cell death.. In previous studies, increased ROS