95
INTRODUCTION
Organ transplantation has been widely used to treat a variety of chronic liver diseases including inborn errors of metabolism, liver cirrhosis and hepatoma. However limitations in the trans- plantation therapy such as high cost, donor shortage, immune rejection and other complications call for the development of alternative treatments. A stem cell therapy, if possible, will have a tremendous advantage over the traditional manipulations in that introduced stem cells can permanently repopulate degenerating hepatocytes in the patient’s liver. Scientists have searched for a reliable cell source that can differentiate into functional hepatocytes, in candidates including adult liver stem cells, bone marrow stem cells and reversibly immortalized heaptocytes.1-3 Unfortunately each of these cell types has its own shortcomings, such as the risk of tumorigenesis in using immortalized cells and the limited proliferation capacity of so- matic stem cells, let alone the problem of MHC compatibility.
ES cells derived from the inner cell mass of mammalian blastocyst are well known for their potential to maintain the undifferentiated state throughout an extended number of passages.4,5 Upon proper stimulation ES cells differentiate into various lineage of all three germ layers, thus can serve as a powerful resource of cell replacement therapy.6 For example, transplantation of neural cells derived from mouse ES cells successfully rescued defective neurons in the central nervous system, proving their potential value in the stem cell therapy.7,8 More recently ES cells have been shown to differentiate into insulin-secreting β-cells, which rescued the diabetic animal.9-11 Furthermore the development of human ES cell lines has opened a new potential usage of ES cells as an excellent source for cell replacement therapy in various human diseases.12,13 Together with the technical availability in the cloning of Purpose: Embryonic stem (ES) cells have been regarded as
a powerful resource in cell replacement therapy. In recent reports, mouse ES cells have been successfully applied to the treatment of spinal cord injuries, hereditary myelin disor- ders of the central nervous system and diabetes mellitus.
Various liver diseases are another group that could benefit from the availability of stem cell therapy; however, no pre- vious demonstration has been made that shows the differen- tiation of ES cells into hepatocytes.
Methods: To investigate the in vivo differentiation potential of mouse ES cells, we injected ES cells into the splenic cortex of immuno-suppressed nude mice.
Results: In a histological analysis of the teratomas derived from injected ES cells some areas were shown, due to their morphology, to contain typical hepatocytes. The hepatic nature of these cells was further confirmed by immunohisto- chemical assays using the antibody against α-fetoprotein and hepatocyte-specific antibodies. In addition, periodic acid- Shiff staining revealed a small portion of hepatic area in the ES-derived teratoma produced glycogen, implying these cells are functional hepatocytes.
Conclusion: Our case demonstrated for the first time that mouse ES cells can differentiate in vivo into a mixed pop- ulation of hepatocytes with different maturation stati, which could potentially extend the usage of ES cells in cell repla- -cement therapy for various liver diseases. (Korean J HBP Surg 2005;9:95-101)
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ Key Words: Stem Cell Transplantation
Cell Differentiation Mice
Stem Cells Hepatocytes
In Vivo Differentiation of Mouse Embryonic Stem Cell into Hepatocytes
Department of Surgery, Hanyang University College of Medicine, Seoul, 1Department of Surgery, Soonchunyang University College of Medicine, Seoul, 2Department of Surgery,
Hallym University College of Medicine, Chuncheon, Korea
Kyeong Geun Lee, M.D., Kwang-Soo Lee, M.D., Hwon Kyum Park, M.D., Dongho Choi, M.D.1 and Han Joon Kim, M.D.2
Correspondence : Kwang-Soo Lee, Department of Surgery, Hanyang University College of Medicine, 17 Haengdang-dong, Seongdong- gu, Seoul 133-792, Korea. (Tel) +82-2-2290-8448, (Fax) +82-2- 2281-0224, (E-mail) hepafel@hanyang.ac.kr
This paper had been presented in 2003 autumn meeting of the Korean Society of Transplantation.
mammalian cells, it is now conceivable to generate a patient’s own ES cell line for developing desired stem cells.6
Experimentally mouse ES cells have been shown to differentiate into many specialized cell types including neural cells, cardiac and skeletal muscle cells, hematopoietic cells, adipocytes, chondrocytes, and osteoclasts.14-20 During in vitro differentiation ES cells express endoderm markers such as α- fetoprotein, albumin, and hepatocyte nuclear factor 3 (HNF3), suggesting their potential to differentiate into hepatocytes.21-23 However there has been no direct evidence made to date demonstrating the differentiation of ES cells into hepatocytes in vivo. To endorse the potential usage of ES cells in the cell replacement therapy of liver diseases, it is important to verify their ability to differentiate into functional hepatocytes. Here we report the first demonstration that mouse ES cells can differen- tiate into functional hepatocytes when injected into nude mouse.
MATERIALS AND METHODS 1. Cell culture
The 129/SvJ ES cell line Pro, a sub-cell line of J1 ES cells originally developed in Dr. Rudolph Jaenisch’s laboratory, was kindly provided by Dr. Hee-Sub Shin (POSTECH). Cells were maintained on the feeder of mitomycin-C treated primary mouse embryonic fibroblasts in Dulbecco’s modified Eagle’s medium supplemented with 15% fetal bovine serum (FBS) (Gibco BRL, Gaithersburg, USA), 0.1 mM β-mercaptoethanol, 1,000 units/ml leukemia inhibitory factor (LIF)(Gibco BRL, Gaithersburg, USA), and 1X nonessential amino acid. The ES cells received at passage 10 were passaged 4 more times before freezing in liquid nitrogen. Frozen vials of ES cells were thawed and passed three more times before transplantation. The feeder fibroblasts were prepared from C57BL/6J embryos of 13.5 to 16.5 day post coitum. Briefly, after removal of soft tissues including heart, liver and other viscerae, the remains of embryo were minced in 2 ml of typsin-EDTA solution (0.05%
trypsin and 0.5 mM EDTA) and passed through a 200μm mesh. After centrifugation, the cell pellets were resuspended in DMEM with 10% FCS, cultured again until confluent, and resuspended in 2ml freezing medium. The frozen feeder ali- quots were thawed before each use and cultured until confluent, and treated with 10μg/ml mitomycin in DMEM with 10%
NCS for 3 hours in 37oC, washed three times with PBS, and plated on a gelatin-coated dish as a feeder layer.
2. Animals
Female nude mice of BALB/c genetic background were
obtained from Korea Food and Drug Administration. 129/SvJ mice were purchased from The Jackson Laboratory (Bar Har- bor, USA). All mice were maintained on a 12 hour light and 12 hour dark cycle, and kept in a pathogen-free environment.
3. ES cell transplantation
Mice were anesthetized by intraperitoneal injection of Avertin solution, the spleen was exteriorized through a 1cm incision on the left flank, and 106 undifferentiated ES cells suspended in 100μl of PBS were injected into the inferior pole of the spleens using a 29 gauge needle. Equal number of ES cells were also injected into the hind leg muscle.
4. Analysis of ES cell-derived teratoma
Three weeks after the transplantation of ES cells, spleens were removed from the injected nude mice and fixed in 4%
paraformaldehyde or 10% formaldehyde. Sections were pre- pared and stained with hematoxylin and eosin (H&E) for histological examination.
5. Immunohistochemistry
The anti-hepatocyte antibody HEP-PAR, a mouse monoclo- nal antibody raised against human hepatocytes, and horseradish peroxidase (HRP)-conjugated secondary antibody against mouse IgG were purchased from DAKO (Copenhagen, Denmark). The anti-hepatocyte antibody was diluted 40 times before use. The rabbit polyclonal antibody against human α-1-fetoprotein was purchased from Quartett (Berlin, Germany) and used without dilution. mNAT antibody was diluted 400 times before use.
Immunostaining was carried out with EnVision kit (DAKO, Copenhagen, Denmark) following the manufacturer’s protocol.
Briefly, the deparaffinized sections were microwaved in target retrieval buffer for 20 minutes and blocked for the endogeneous peroxidase by incubating in the peroxidase block solution for 10 minutes. The sections were incubated with primary antibody for 30 minutes at room temperature and washed with PBS three times for 5 minutes each. The sections were then incubated in EnVision solution for 30 minutes and washed as before, and then treated with chromogenic substrate solution for 10 minutes. Each section was counter-stained with hematoxylin to reveal cellular structures.
6. Periodic acid-Shiff histochemical staining
Deparaffinized tissue sections were immersed in periodic acid Solution for 5 minutes at room temperature and rinsed 3 times with distilled water. The sections were treated with Shiff’s reagent (Sigma, Saint Louis, USA) for 15 minutes at
room temperature and washed in running tap water for 5 minutes. After a 90 second counterstaining with hematoxylin, sections were dehydrated, cleared and mounted.
RESULTS
To determine whether ES cells differentiate into hepatocytes
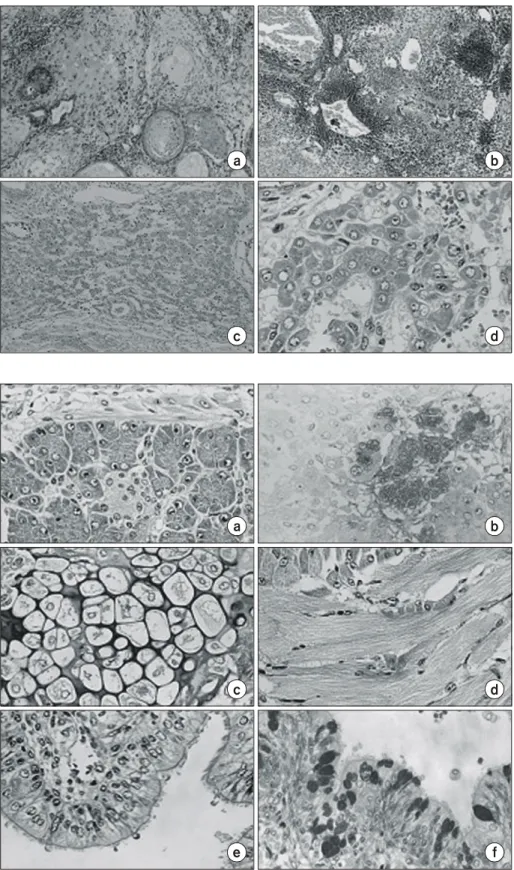
Fig. 2. Various types of cells found in an ES teratoma formed in the nude mouse spleen (×400). (a) Pancreatic acinus cells (H&E). (b) Pancreatic β- islet cells immunostained with anti- insulin antibody and counter-stained with hematoxylin. (c) Chondrocytes (H&E). (d) Striated muscle (H&E).
(e) Gut-like cells (H&E). (f) Gut-like cells stained with PAS.
d c
e f
a b
Fig. 1. Histological sections (H&E) of ES cell-derived teratomas. ES cell- derived teratoma formed in the hind leg muscle (a, ×100) and in the liver (b, ×100) of syngeneic 129/SvJ mice.
ES cell-derived hepatocyte-like cells in the teratoma of nude mouse spleen (c, ×100; d, ×400).
a b
c d
in vivo, we introduced undifferentiated mouse ES cells derived from the 129/SvJ strain into the spleen and hind leg muscle of syngeneic mice. Three weeks later teratomas formed from the transplanted ES cells were histologically analyzed to determine the types of cells present in the tumors. The teratomas were mainly composed of neuroectodermal cells and primitive neuroectodermal cells in the hind leg and spleen, respectively (Fig. 1a, 1b) and none of the ES cell-derived teratomas was found the cells differentiated into hepatocytes in the syngeneic 129/SvJ mouse. Therefore, the ES cells were introduced into the spleen of immune-suppressed nude mice. In contrast to the teratomas developed in the syngeneic mice, the teratoma formed in the inferior pole of nude mouse spleen contained cells showing morphological characteristics of hepatocyte (Fig. 1c, 1d). Similar experiments were repeated more than three times and produced comparable results, indicating that the microenvironment in which the ES cells were transplanted had effects on the differentiation of the cells.
The hepatocyte-like cells in the spleen teratoma with typical large nucleus and abundant cytoplasm were arranged in a cord-like, sinusoidal structure (Fig. 1d). The ES cell-derived
teratoma in the nude mouse spleen also contained other types of differentiated cells including, chondrocytes, gut-like epithe- lium, pancreatic cells, and striated muscle cells (Fig. 2a∼f).
Primitive thyroid follicular cells, salivary gland-like cells, and gut-like epithelium were positively stained by periodic acid- Shiff (PAS), confirming their identity. In particular, some pan- creatic cells exhibited β-islet-like structures, which were pos- itively immunostained with an anti-insulin antibody (Fig 2b).
The hepatic nature of cells in the spleen teratoma was further confirmed by an immunohistochemistry analysis using hepato- cyte-specific antibodies. An anti-hepatocyte antibody, HEP- PAR, has been widely used for the detection of human hepato- cytes in various metastasized tumors.24,25 Since the target antigen of HEP-PAR has not yet been identified, we tested the antibody on normal mouse livers from various developmental stages. HEP-PAR antibody specifically recognized adult hep- atocyte but not embryonic hepatocytes from 13.5 and 16.5 dpc, indicating that the antigen of this antibody is expressed in matured hepatocytes (Fig. 3a, 3b). The HEP-PAR antibody specifically immunostained the ES cell-derived hepatocytes with sinusoidal structure with high affinity (Fig. 3c, 3d), indi-
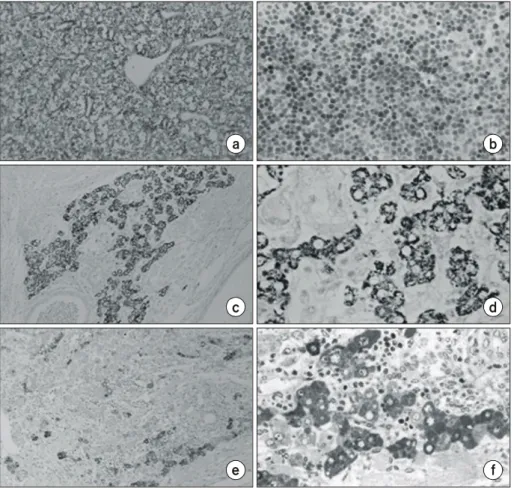
Fig. 3. Confirmation of the hepatic nature of cells in the ES teratoma.
Cells were immunostained with li- ver-specific antibodies and counter- stained with hematoxylin. Sections of normal adult mouse liver (a, ×400) and E 13.5d embryo liver (b, ×400) were immunostained with anti-hepa- tocyte antibody, HEP-PAR. ES cell- derived teratoma was immunostained with HEP-PAR (c, ×100; d, ×400).
ES cell-derived teratoma immuno- stained with anti-α-fetoprotein (e, × 100; f, ×400).
a b
d c
e f
cating that the hepatic area in the teratoma contained matured hepatocytes. To further confirm the hepatic lineage of the ES cells, an antibody against α-fetoprotein, a well-known marker of embryonic hepatocyte, was used in the immunostaining of the teratomas. The α-fetoprotein antibody recognized some but not all cells in the hepatic area (Fig. 3e, 3f), suggesting that the hepatocytes in this area consisted of heterogeneous pop- ulation of hepatocytes.
To study whether the ES cell-derived hepatocytes produce functional hepatocyte-enriched proteins, we analyzed the ex- pression of mouse N-system amino acid transporter (mNAT), a membrane-bound protein produced in adult liver.26 Anti- mNAT antibody interacted with a small number of cells in the hepatic area of the teratoma (Fig. 4a, 4b). In addition, a small portion of the ES cell-derived hepatocytes stained with PAS which recognizes mucopolysaccharide (Fig. 4c, 4d). PAS has been used to determine the storage of glycogen, which is abundant in functional hepatocytes of adult liver. These results lead to an implication that some of the ES cell-derived hepato- cytes are functionally mature, and this is in agreement with the heterogeneous distribution of cells in various differentiation stages observed from the immunohistochemcal analysis of he- patic markers.
DISCUSSION
With its pluripotency and proliferation, ES cell is considered as one of the best cell sources for the cell replacement therapy.
Recently ES cells have been reported to differentiate in vitro into cells of endodermal origin that express proper endodermal and hepatic markers.22 However no histological identification has yet been made either in vitro or in vivo for these hepato- cyte-like cells derived from ES cells. Our results provide the first histological example demonstrating the potential of ES cells to differentiate in vivo into hepatocytes expressing proper markers. Not only the ES cell-derived hepatocyte expressed hepatocyte-specific markers, some of these cells exhibit charac- teristic functions of a normal liver cell, e.g. the expression of N-system amino acid transporter, mNAT and the cytoplasmic accumulation of glycogen.
It appears that the microenvironment surrounding the intro- duced ES cells has a great influence on the directions of their differentiation. While the ES cells injected into the syngeneic 129/SvJ mice mainly differentiated into neuroectodermal lineage, the cells injected into immuno-suppressed nude mice underwent differentiation into diverse cell types including hepatocytes. Even the ES cell-teratomas developed in the leg muscle of nude mice did not contain any hepatocyte-like cells but mostly consisted of the cells of ectodermal origin (data not shown). This result confirmed that the microenvironment signals coming from the surrounding spleen affected the hepatic differentiation of injected ES cells. It will be important to identify these factors and understand the way how they govern the process of ES cell differentiation.
Interestingly, the cells in the hepatic area of ES cell-derived teratoma appears to express differenential level of hepatic Fig. 4. Functional analysis of ES cell-derived hepatocytes in the tera- toma by immunohistochemistry and PAS staining (×400). ES cell-derived hepatocytes (a) and adult normal liver (b) immunostained with anti-mNAT antibody. Arrow indicates the cells positive to the antibody. Sections were counter-stained with hematoxy- lin. ES cell-derived hepatocytes (c) and adult normal liver (d) stained with PAS. Arrows indicate the cells stained with PAS.
a b
c d
markers, α-fetoprotein and HEP-PAR antigen, and also varied in the amount of cellular storage of glycogen, indicating that a given area is populated with hepatic cells in varying stages of differentiation. During the organogenesis of embryo, the liver bud formed by the proliferation of gut endodermal cells is populated mostly by hepatoblasts expressing cytokeratins common to both hepatocytes and bile duct cells, then the subsequent expression of α-fetoprotein and albumin by most hepatic cells identifies hepatocytic lineage. During these lineage establishment α-fetoprotein is transiently expressed. Based on our results the 13.5 d mouse embryonic liver does not express HER-PAR antigens, while the antigen was detected in the hepatocyte lineage cells of neonatal mouse liver, 2 week-old and 2 month-old mouse liver but not in the bile duct lineage cells. Since a large number of metabolic enzymes are induced within the hepatocytes during the perinatal period, these results suggest that HER-PAR antigen is expressed only in the mature hepatocyte and HER-PAR antigen seems to be one of the molecular marker expressed at the early stage of mature hepatocyte. The hepatic area of the teratoma, therefore, appear to comprise immature hepatocytes, mature hepatocytes, and fully functional hepatocytes, and we could not morphologically detect any bile duct-like cells in the ES cell-derived teratoma.
The apparent heterogeneity in the differentiation status indicates that these ES cell-derived hepatocytes seem to be in the process of completing their maturation, very likely following the pattern of normal hepatic cell differentiation during liver development.
In order to study the functional potential of the ES cell- derived hepatocytes, it is conceivable to isolate the hepatic cells from the teratoma by fluorescent activated cell sorter using a hepatocyte-specific cell surface marker, to transplant the purified cells into mouse models of liver metabolic diseases, and to examine the effect on the treated mice. Information obtained in the animal-based study will eventually help re- searchers to develop ways to utilize human ES cells in the cell replacement therapy for patients suffering from various liver diseases in a near future.
REFERENCES
1) Kobayashi N, Fujiwara T, Westerman KA, et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science 2000;287:1258-1262.
2) Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science 1999;284:
1168-1170.
3) Sell S. Is there a liver stem cell? Cancer Research 1990;50:
3811-3815.
4) Evans MJ, Kaufman MH. Establishment in culture of pluri- potential cells from mouse embryos. Nature 1981;292:154-156.
5) Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by terato- carcinoma stem cells. Proc Natl Acad Sci USA 1981;78:
7634-7638.
6) Solter D, Gearhart J. Putting stem cells to work. Science 1999;283:1468-1470.
7) Brüstle O, Jones KN, Learish RD, et al. Embryonic stem cell- derived glial precursors: a source of myelinating transplants.
Science 1999;285:754-756.
8) McDonald JW, Liu X, Qu Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 1999;5:1410-1412.
9) Assady S, Maor G, Amit M, et al. Insulin production by human embryonic stem cells. Diabetes 2001;50:1691-1697.
10) Lumelsky N, Bondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001;292:1389-1394.
11) Soria B, Roche E, Berná G, et al. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozocin-induced diabetic mice. Diabetes 2000;49:157-162.
12) Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro.
Nat Biotechnol 2000;18:399-404.
13) Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;
282:1145-1147.
14) Dani C, Smith AG, Dessolin S, et al. Differentiation of em- bryonic stem cells into adipocytes in vitro. J Cell Sci 1997;
110:1279-1285.
15) Doetschman TC, Eistetter H, Katz M, et al. The in vitro development of blastocyst-derived embryonic stem cell lines:
formation of visceral yolk sac, blood islands and myocardium.
J Embryol Exp Morph 1985;87:27-45.
16) Klug MG, Soonpaa MH, Koh GY, et al. Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J Clin Invest 1996;98:216-224.
17) Kramer J, Hegert C, Guan K, et al. Embryonic stem cell- derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev 2000;92:193-205.
18) Nakano T, Kodama H, Honjo T. Generation of lymphohemato- poietic cells from embryonic stem cells in culture. Science 1994;265:1098-1101.
19) Wobus AM, Holzhausen H, Jäkel P, et al. Characterization of a pluripotent stem cell derived from a mouse embryo. Exp Cell Res 1984;152:212-219.
20) Yamane T, Kunisada T, Yamazaki H, et al. Development of osteoclasts from embryonic stem cells through a pathway that is c-fms but not c-kit dependent. Blood 1997;90:3516-3523.
21) Abe K, Niwa H, Iwase K, et al. Endoderm-specific gene expression in embryonic stem cells differetiated to embryoid
ꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏꠏ bodies. Exp Cell Res 1996;229:27-34.
22) Hamazaki T, Liboshi Y, Oka M, et al. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett 2001;
497:15-19.
23) Levinson-Dushnik M, Benvenisty N. Involvement of hepato- cyte nuclear factor 3 in endoderm differentiation of embryonic stem cells. Mol Cell Biol 1997;17:3817-3822.
24) Demetris AJ, Seaberg EC, Wennerberg A, et al. Ductular re- action after submassive necrosis in humans: special emphasis on analysis of ductular hepatocytes. Am J Pathol 1996;149:
439-448.
25) Wennerberg AE, Nalesnik MA, Coleman WB. Hepatocyte par- affin 1: a monoclonal antibody that reacts with hepatocytes and can be used for differential diagnosis of hepatic tumors.
Am J Pathol 1993;143:1050-1054.
26) Gu S, Roderick HL, Camacho P, et al. Identification and char- acterization of an amino acid transporter expressed differen- tially in liver. Proc Nat Acad Sci USA 2000;97:3230-3235.
25) Wennerberg AE, Nalesnik MA, Coleman WB. Hepatocyte par- affin 1: a monoclonal antibody that reacts with hepatocytes and can be used for differential diagnosis of hepatic tumors.
Am J Pathol 1993;143:1050-1054.