1
Proteasome 억제에 의한 P53의 발현과 미토콘드리아 막 전압의 소실로 TRAIL에 저항하는 폐암세포의 사멸 강화
설재원·박상열*
전북대학교 헬스케어 기술개발사업단, 수의과대학 생체안전성연구소 (게재승인: 2009년 2월 10일)
The proteasome inhibition enhances apoptosis by P53 expression and the dissipation of mitochondrial transmembrane potential
in TRAIL-resistant lung cancer cells
Jae-Won Seol, Sang-Youel Park*
Center for Healthcare Technology Development, and Bio-safety Research Institute, College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea
(Accepted: February 10, 2009)
Abstract : The ubiquitin-proteasome mediated protein degradation pathway plays an important role in regulating both cell proliferation and cell death. Proteasome inhibitors are well known to induce apoptosis in various human cancer cell lines. We investigated the effect of combined treatment with proteasome inhibitor and TRAIL, and a possible mechanism of the enhancing apoptosis by the both treatment, on TRAIL-resistant non-small cell lung cancer. A549 cells were exposed to the N-Acetyl-Leu-Leu-Norleu-al (ALLN) as a proteasome inhibitor and then treated with recombinant TRAIL protein. In A549 cells under proteasome inhibition conditions by pretreatment with ALLN, TRAIL treatment significantly decreased cell viability compared to that ALLN and TRAIL alone treatment. Also, the both treatment induced cell damage through DNA fragmentation and p53 expression. In addition, the combined treatment of both markedly increased caspase-8 activation, especially the exposure for 2 h, and Bax expression and induced the dissipation of mitochondrial transmembrane potential in A549 cells. Taken together, these findings showed that proteasome inhibition by ALLN enhanced TRAIL-induced apoptosis via DNA degradation by activated P53 and mitochondrial transmembrane potential loss by caspase-8 activation and bax expression. Therefore, our results suggest that proteasome inhibitor may be used a very effectively chemotherapeutic agent for the tumor treatment, especially TRAIL-resistant tumor cell.
Keywords : ALLN, caspases, MTP, P53, TRAIL
서 론
세포내에서일어나는단백질분해과정중약
80%
는
ubiquitin
에의해표지된후proteasome
에의해세포 질과 핵에서단백질이분해되며,
이과정을ubiquitin- proteasome system(UPS)
라한다.
이러한UPS
를통한세포내단백질의분해는세포의증식과사멸을조절하는
데중요한역할을하는것으로알려져있지만
, UPS
에의한세포의증식과사멸등의상반되는영향에대해서 는아직까지많은논란이되고있다
[17, 31].
Proteasome
은multicatalytic protease complex
로서lysosome
에의존하지않고ubiqutin
과ATP
에의존하여*Corresponding author: Sang-Youel Park
College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea [Tel: +82-63-270-3886, Fax: +82-63-270-3780, E-mail: sypark@chonbuk.ac.kr]
빠르게단백질을분해하는세포내기본적인장치를말 한다
[6, 9, 20].
최근에proteasome
을통해분해되는많은단백질이세포사멸
(apoptosis)
과정에관련된물질들임이밝혀지면서
ubiquitin-proteasome system
이세포사멸의조절에중요한역할을하는것으로생각되고있 다
.
또한proteasome
에대한억제제를사용하여일부암 환자들에게 좋은치료효과를보인다고보고하였다[3, 14, 16].
그러나proteasome
의억제제는세포들의종류에 따라세포사멸의억제및유도의차이를보이고있다[7, 29].
TRAIL
은tumor necrosis factor(TNF)-related apoptosis inducing ligand
의 약어로써TNF family
중에type II transmembrane cytokine molecule
로분류되며, ligand-type
의세포사멸을 유도하는 단백질을의미한다
[4, 15].
TRAIL
은세포표면에존재하는TRAIL
관련수용체들즉
, Death Receptor-4(DR-4), Death Receptor-5(DR-5), Decoy Receptor
그리고Decoy Receptor-2
와결합함으로 써세포사멸신호전달계를조절및활성화시켜세포사멸을유도하는것으로알려져있다
. TRAIL
은특히암세포에만특이적으로작용하여세포사멸을유도하는것
으로보고되어있으며
[10, 34],
정상세포에서는아무런독성이없는것으로나타나
,
새로운종양치료제로서많은연구가진행중이다
.
Calpain
억제제이자proteasome
억제제로알려진N- Acetyl-Leu-Leu-Norleu-al(ALLN)
은세포사멸을유도하는것으로알려져있으며
,
특히cyclin-dependent protein kinase inhibitor
인P21WAF1
과p53
등과같은세포내단 백질의양을증가시켜세포사멸을유도한다고보고되었지만
[1, 19],
비소세포폐암을포함한여러종양세포에서의 효과와그기전에 대한연구는부족한실정이
다
.
또한proteasome
억제제가 다양한 종양세포에서TRAIL
과의병행처리시세포사멸을증가시킨다는보고는있지만
[8, 13, 22, 26],
비소세포폐암에서ALLN
과TRAIL
의처리에대한효과와그기전에관한연구는보고된바가없다
.
본연구에서는비소세포폐암세포인
A549
세포내에proteasome
의억제가세포사멸을유도하는지확인하였으며
,
세포내에proteasome
의억제후재조합된TRAIL
단백질의병행처리가
TRAIL
에저항하는A549
세포의사멸을증가시킬수있는지와그세포사멸유도경로 를조사하였다
.
재료 및 방법
Cell culture
본실험에서사용된
A549
세포주는ATCC(USA)
로부터공급받아사용하였다
.
세포의성장 유지를위해서100
µg/ml gentamicin, 100
µg/ml penicillin-streptomycin
과10%(V/V) fetal bovine serum
이첨가된RPMI
배지에5%
CO
2를 공급하고37
oC
로 배양 시켰다. ALLN(Sigma, USA)
은10 mM
농도로dimethylsulphoxide
에녹여보관한후사용하였다
. Cell viability test
세포를
12-well plate
에1.0 × 10
4 이되게각well
에 넣은 다음5% CO
2,37
oC
상태에서24
시간 배양시켰다
. 12-well plate
에서증식한세포에ALLN(10
µM) 1
시간 동안전처리한 후재조합
TRAIL(100 ng/ml)
단 백질을[32]
처리하여3
시간 동안 더배양하였다.
세포의생존능측정은
crystal violet
염색방법에의해검 사할수있었고세포의형태를 현미경에서검사한 후 사진촬영을하였다.
세포의생존능측정은세포를30%
ethanol
과3% formaldehyde
가 들어있는0.5% crystal violet
으로실온에서10
분간염색하고흐르는물에서4
회세척한후건조하였다
.
건조된후에세포를1% SDS
용액으로 용해시켜서
550 nm
에서흡광도를 측정하였으며
,
대조군을100 %
로정하고세포의생존능을 결정하였다
.
DNA fragmentation test
Tissue culture dish
에세포를배양한후ALLN(10
µM)
을
1
시간동안전처리하고재조합TRAIL(100 ng/ml)
단 백질을2
시간동안처리하였다.
세포를phosphate-bufferd saline(PBS)
으로세척한후0.5 ml DNA lysis buffer(10 mM Tris, 1 mM EDTA, 0.2% TRITON X-100)
와0.5 mg/
ml proteinase K(Sigma-Aldrich, Germany)
를넣고50
oC
에서
1
시간동안배양시켰다.
용해물에동량의phenol
을 넣어준후12,000 rpm
에서5
분간원심분리하였다.
상층 액을모아서3 M sodium acetate
를시료의10%
가되게넣고
cold ethanol
을시료의양에2
배를넣어DNA
를침 전시켰다.
원심분리후pellet
을vacuum drier
에건조시 켰고, TE buffer(10 mM Tris, 1 mM EDTA, pH 7.4)
를넣어용해시켰다
. Loading buffer(Sigma, USA)
를넣은후DNA 3
µg/ml
을1.2%(w/v) agarose gel
을이용하여75 V
에서
2
시간동안분리하였고, ethidium bromide
를이용하여
UV imaging illuminator(Gel Doc 1000 Darkroom;
Bio-Rad, USA)
로확인하였다. Western blot assay
Tissue culture dish
에세포를배양한후ALLN(10
µM)
을
1
시간동안전처리하고재조합TRAIL(100 ng/ml)
단 백질을0.5, 1
그리고2
시간동안처리하였다.
배양된세포를
scraper
를이용하여모은 다음PBS
로세척한후2,000 rpm
에서10
분간원심분리해서상층액을제거하였다
.
모아진세포에용해액(25 mM HEPES(pH 7.4), 100 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 0.1 mM DTT, and protease inhibitor mixture)
을넣어부유시킨 후얼음에30
분간방치하고sonication
하였다.
정량을통해시료에서일정한양의단백질을취한후단백질분자량
marker
와함께
SDS-PAGE
를하여단백질을분리하고nitro- cellulose membrane
에transfer
시켰다. 1
차antibody
와탐 식자가결합된2
차antibody
를처리한후ECL solution
(Pierce, USA)
을처리하고암실에서현상하여확인하였다
.
본실험에사용한antibody
인p53
은Santa Cruz(USA)
에서
, caspase-8
과bax
는BD Pharmingen(USA)
에서구입하여사용하였다
. Western blot
에의해얻은결과는laser scanning densitometry(Bio-Rad Laboratories, USA)
를이 용하여분석하였다.
Evaluation of mitochondrial transmembrane poten- tial (MTP)
MTP
는5,5',6,6-tetrachloro-1,1'3,3'-tetraethybenzimidazolyl- carbocyanine iodide(JC-1)
를이용하여 측정하였다.
먼저6-well plate
에A549
세포를배양한후ALLN(10
µM)
을1
시간전처리한후재조합TRAIL(100 ng/ml)
단백질을 처리하여2
시간동안더배양하였다.
배양후scraper
를이용하여세포를모으고
2,000 rpm
에서5
분동안원심 분리하고PBS
를이용하여2
번세척하였다.
다시10,000 rpm
에서5
분간원심분리한후상층액을버리고10
µM JC-1
이포함된PBS
를500
µl
넣어준후잘혼합시켰다. 37
oC
암실에서30
분동안놓아둔후excitation 490 nm
과
emission 530 nm
에서측정하였으며,
또한형광현미경을이용하여형광촬영을실시하였다
. Statistical evaluation
본실험에서통계학적유의성은
SAS statistical package (release 8.1; SAS Institute, USA)
를이용하여ANOVA
를 실시하고,
p< 0.05, 0.01
이하의 유의성을갖는경우에통계학적차이로인정하였다
.
결 과
A549 세포의 proteasome 억제는 TRAIL이 유도 하는 세포사멸을 강화시킨다.
본연구에서는
proteasome inhibitor
와재조합TRAIL
단백질의처리가
A549
세포에어떠한효과를보이는지확인하기 위하여
, ALLN(10
µM, 4 h)
과TRAIL(100 ng/
ml, 3 h)
을각각처리하였으며,
또한, ALLN(10
µM)
을1
시간동안전처리하고재조합
TRAIL(100 ng/ml)
단백질을처리한후에
3
시간더배양시켰다.
그결과ALLN
과재조합
TRAIL
단백질의단독처리시에는약10-15%
의세포사멸을보였으나
ALLN
의전처리와TRAIL
의병행처리시
A549
세포의사멸이약54%
로강화되는것을확인하였다
(Fig. 1A).
현미경관찰역시ALLN
과TRAIL
의단독처리보다는A549
세포내에ALLN
에의한
proteasome
의억제후TRAIL
의처리가세포의생존 능력을더욱감소시킨다는것을확인하였다(Fig. 1B).
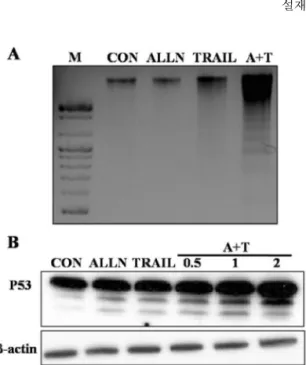
Proteasome의 억제제와 TRAIL의 병행 처리는 DNA 손상과 P53의 증가를 유도한다.
세포사멸의생화학적인특징중하나는세포내
DNA
가큰 조각에서작은 조각으로 갈라지는
DNA frag-
mentation
이다.
이번연구에서는proteasome inhibitor
와 재조합TRAIL
단백질의처리가A549
세포에서DNA fragmentation
을 보이는지 조사하였다. A549
세포에ALLN(10
µM, 3 h)
과TRAIL(100 ng/ml, 2 h)
을각각처리하였으며
,
또한, ALLN(10
µM)
을1
시간동안전처리하고재조합
TRAIL(100 ng/ml)
단백질을처리한후에2
Fig. 1. Proteasome inhibition enhances TRAIL-induced
apoptotic cell death in A549 cells. (A) A549 cells cultured
in 12-well plate were pretreated with ALLN (10
µM) for
1 h, and then further co-incubated with recombinant TRAIL
protein (100 ng/ml) for additional 3 h. Cell viability was
determined by crystal violet staining. Viability of control
cells was set at 100%, and viability relative to the control
is presented. The experiments were performed at triplicate,
at least twice. Standard error is expressed as
**p< 0.01
according to the student’s
t-test. (B) Cell morphology under
the conditions described in (A) was photographed under
microscope.
시간더배양시킨 다음
DNA
를추출하여DNA frag- mentation
정도를확인하였다.
그결과ALLN
과TRAIL
의병행처리시
DNA
가큰조각에서작은조각으로분절이증가하는것을확인할수있었다
(Fig. 2A).
다음은
DNA
손상에따른P53
단백질의발현정도를확인하기위하여
Western blotting
을실시하였다.
그결과
P53
단백질의발현이ALLN
과TRAIL
의병행처리시증가하는것을보였으며
, TRAIL
의처리시간에비례하여증가하는것을확인할수있었다
(Fig. 2B).
이러한결과는
proteasome
억제와TRAIL
의처리시DNA
의손상에따른
p53
의활성이증가하여세포사멸이강 화되는것을보여주는것이다.
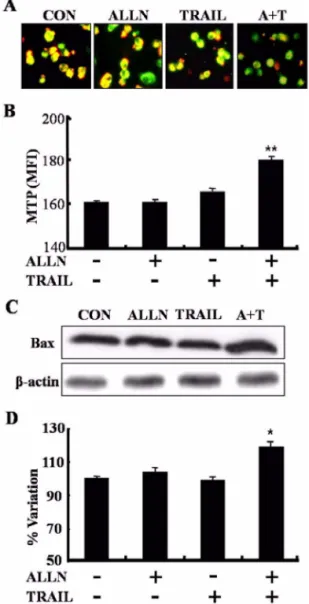
Caspase-8의 활성과 미토콘드리아 막전압의 소실 에 의해 A549 세포 사멸이 강화된다.
다음으로세포사멸개시인자인
caspase-8
이ALLN
과TRAIL
의병행처리시어떠한변화를보이는지조사하기위하여
Western blotting
을실시하였다.
그결과ALLN
과
TRAIL
의병행처리시caspase-8
의활성이ALLN
과TRAIL
의단독처리보다증가되는것을확인할수있었으며
, TRAIL
의처리 시간에의존적으로활성화된caspase-8
이더증가되는것을확인할수있었다(Fig. 3).
최근에세포사멸과관련하여미토콘드리에서여러가 지방법을통하여세포사멸을유도하는것으로알려지 고있다
.
본연구에서는A549
세포에ALLN
과TRAIL
을 처리하였을때미토콘드리아의기능변화를확인하기위하여
, JC-1
을사용하여미토콘드리아막전압(MTP)
의변화정도를측정하였다
.
형광현미경관찰시대조군에 서정상적인MTP
를나타내는붉은색이많이보이는반면에
, ALLN
과TRAIL
의병행처리시MTP
의소실을 의미하는녹색이현저히많은것을확인할수있었다(Fig. 4A).
또한형광광도계측정결과녹색의형광값이ALLN
과TRAIL
의병행처리시더많이증가하는것을볼수있었다
(Fig. 4B).
또한bcl2 family
중에하나인Bax
의발현을확인해본결과ALLN
과TRAIL
의병행처리시세포내많이증가가되는것을확인할수있 었으며
(Fig. 4C), laser scanning densitometry
의결과는이 를지지해준다(Fig. 4D).
이상의결과는ALLN
과TRAIL
의병행처리가
caspase-8
의활성과세포내Bax
의발현 을유도하고이에따른미토콘드리아막전압의소실을야기하여
A549
세포의사멸을강화시킨다는것을보여주는것이다
. Fig. 2. Pretreatment of proteasome inhibitor with TRAIL
induce DNA fragmentation and p53 expression. (A) A549 cells in tissue culture dish (60 × 15 mm) were pretreated with ALLN (10
µM) for 1 h, and then further co-incubated with recombinant TRAIL protein (100 ng/ml) for additional 2 h. DNA (3
µg/ml) was loaded on 1.2% agarose for 2 hours at 75 V. DNA laddering was visualized under UV light by staining the agarose gel with ethidium bromide.
(B) A549 cells were pretreated with ALLN for 1 h, and then further co-incubated with or without recombinant TRAIL protein (100 ng/ml) for additional 0.5, 1 and 2 h.
Whole cell lysates were prepared and protein sample (40
µg/ml) were separated on SDS gel, analyzed the expression of p53 by Western blotting analysis.
β-actin indicates a non-specific protein band used to ensure equal protein loading.
Fig. 3. Caspase-8 activation was increased by pretreatment
of proteasome inhibitor with TRAIL. A549 cells were
pretreated with ALLN for 1 h, and then further co-incubated
with or without recombinant TRAIL protein (100 ng/ml) for
additional 0.5, 1 and 2 h. Whole cell lysates were prepared
and protein sample were separated on SDS-PAGE, analyzed
the activation of caspase-8 by Western blotting analysis.
고 찰
Bortezomib, MG132, PS-341
그리고ALLN
과 같은다양한
proteasome
억제제들이많은세포들에서세포사멸을유도한다고보고되었으며
[24, 25, 27],
이러한proteasome
억제제에의해TRAIL
이유도하는 세포사 멸이강화된다고알려져있다[8, 22, 26, 35].
본실험에서는비소폐암세포인
A549
세포에먼저proteasome
억제제인
ALLN
을전처리한후재조합된TRAIL
단백질을처리하여그세포사멸효과와그기전에대하
여조사 하였다
. ALLN
과TRAIL
의단독처리에 비해ALLN
의전처리후TRAIL
의처리가A549
세포의사 멸을증가시킨다는것을확인할수가있었으며,
이것은
A549
세포내proteasome
의억제가TRAIL
이유 도하는세포사멸을더욱강화시킬수있다는것을보여 주는것이다.
일반적으로
tumor suppressor
로알려진P53
은세포주 기와세포사멸을조절함으로써세포내항상성을유지 하는단백질로알려져있다.
평상시에는mdm2
라는단백질과결합한상태로존재하는데
,
세포가DNA
의손상 을받거나저산소상태의스트레스에노출되면활성이 증가하는것으로알려져있다[11, 12].
사람의암세포에서
P53
의과발현에의한TRAIL
의세포사멸효과가강화된다는보고가있으며
[18],
사람의폐포상피세포에대한
proteasome
억제제인MG132
의처리가세포내활성산소종
(reactive oxygen species)
을형성하여P53
의활성을유도하고
,
이로인해TRAIL
이유도하는세포사멸을증가한다는보고가있다
[5].
본실험에서는A549
세포에
proteasome
억제와TRAIL
의 처리시DNA fragmentation
이일어나는것을확인할수있었으며,
또 한Western blotting
결과A549
세포내에P53
단백질의발현이
ALLN
의전처리와TRAIL
의시간별병행처리시
TRAIL
의처리시간에비례하여증가하는것을확인할수있었다
.
이러한결과는proteasome
억제와TRAIL
의처리시
A549
세포내에DNA
분절에따른DNA
의 손상이일어나며,
이에따른P53
의발현이증가하여세포사멸을더욱강화시키는것으로생각된다
.
TRAIL
이유도하는세포사멸은TRAIL
수용체인DR- 4
와DR-5
와의 결합에 의해death-inducing signaling complex(DISC)
가 형성됨으로서 시작되며, DISC
에는FADD
와caspase-8
등도관련되어있다[30, 36].
이중 에서caspase-8
은TNF-
α또는Fas ligand
와같은세포사멸유도물질에의해활성화되며일련의 다른
caspase
를 활성화시키면서 세포사멸을 유발시킨다
[2, 23].
Voortman
등은비소세포폐암에서proteasome
억제제인bortezomib
이caspase-9
뿐만아니라caspase-8
의활성을Fig. 4. Pretreatment of proteasome inhibitor with TRAIL led to loss in mitochondrial transmembrane potential (MTP). (A) A549 cells plated in 6-well were pretreated with ALLN (10
µ
M) for 1 h, and then further co-incubated with recombinant TRAIL protein (100 ng/ml) for additional 2 h. MTP was determined using JC-1 probe. The cells were photographed using a fluoroscope. (B) The green fluorescence intensity was measured under the conditions as described in (A) at 530 nm (emission of JC-1 monomeric form) and 590 nm (emission of JC-1 aggregate) when excited at 490 nm. For the each case, the mean fluorescence intensity values are indicated. (C) A549 cells were treated under the conditions as described in (A). Bax expression was analyzed by Western blotting analysis. (D) Western blotting results were analyzed with image analysis software. Data are expressed as the percentage of sham group protein expression. Standard error is expressed as
*p< 0.05,
**p< 0.01 according to the student’s
t
-test.
β-actin indicates a non-specific protein band used to
ensure equal protein loading.
증가시켜
TRAIL
이유도하는세포사멸을증가시킨다고보고 하였으며
[33],
골육종(osteosarcoma)
세포에서proteasome
억제제인MG132
가caspase-8
과Bax
의발현 을증가시켜세포사멸을유도한다고보고하였다[37].
본실험에서는
A549
세포내에ALLN
처리후TRAIL
의처리시세포내
caspase-8
의활성과Bax
의발현이증가 하는것을 볼수있었으며,
이것은세포내proteasome
억제가
TRAIL
처리에의한caspase-8
의활성을더욱증 가시킨다는것을보여주며,
이로인해Bax
등과같은세 포사멸관련인자들을활성화시켜세포사멸을유발시 키는것으로생각된다.
세포사멸과관련하여최근에미토콘드리아는여러가 지방법을통하여세포사멸을유도하는것으로알려지 고있다
.
세포사멸의신호자극에의하여미토콘드리아 막전압의변화나활성산소종(ROS)
의형성에의하여미 토콘드리아투과성이변화되어작은구멍(pore)
을형성하고이를통하여세포질내에
cytochrome-c
와같은세 포사멸인자들이방출된다[21, 28].
본연구에서는A549
세포에
ALLN
의전처리와TRAIL
을병행처리하였을때미토콘드리아막전압의변화정도를확인하였고
,
그 결과proteasome
억제후TRAIL
의처리시미토콘드리 아막전압이소실되는것을확인할수있었다.
이러한결과는
proteasome
의억제와TRAIL
의처리에의하여미 토콘드리아의활성이떨어져ATP
등에너지대사와관련된인자들의작용이억제되어
A549
세포사멸을더욱강화시키는것으로생각된다
.
결 론
본연구는
TRAIL
에저항성을가진것으로알려진비소세포폐암에서
proteasome
의억제가TRAIL
이유도하는세포사멸을강화시킬수있는지를조사하였으며
,
그 강화된세포사멸유도경로를조사하였다. ALLN
에의 한proteasome
억제후에TRAIL
의처리는A549
세포의생존능을감소시켰으며
,
세포내DNA fragmentation
이일어났다
.
또한세포내P53
의발현과caspase-8
의활성
, Bax
의발현이증가하였고,
미토콘드리아막전압의소실을 야기하였다
.
결론적으로 비소세포폐암에서proteasome
억제가p53
의 발현에 의한DNA
손상과caspase-8
의활성과Bax
의증가를통한미토콘드리아의기능소실에의하여
TRAIL
이유도하는세포사멸을증가시킨다는것을보여주며
,
이러한결과는TRAIL
에저항성을가진종양의치료시
proteasome
억제제인ALLN
등을병행처리함으로서종양의치료효과를극대화시 킬수있을것으로사료된다
.
감사의 글
이논문은교육과학기술부로부터지원받아수행된연 구임
(
지역거점연구단육성사업/
헬스케어기술개발사업단).
참고문헌
1. An WG, Hwang SG, Trepel JB, Blagosklonny MV.
Protease inhibitor-induced apoptosis: accumulation of wt p53, p21WAF1/CIP1, and induction of apoptosis are independent markers of proteasome inhibition. Leukemia 2000, 14 , 1276-1283.
2. Boldin MP, Goncharov TM, Goltsev YV, Wallach D.
Involvement of MACH, a novel MORT1/FADD- interacting protease, in Fas/APO-1- and TNF receptor- induced cell death. Cell 1996, 85 , 803-815.
3. Brooks AD, Ramirez T, Toh U, Onksen J, Elliott PJ, Murphy WJ, Sayers TJ. The Proteasome Inhibitor Bortezomib (Velcade) Sensitizes Some Human Tumor Cells to Apo2L/TRAIL-Mediated Apoptosis. Ann N Y Acad Sci 2005, 1059 , 160-167.
4. Cha SS, Kim MS, Choi YH, Sung BJ, Shin NK, Shin HC, Sung YC, Oh BH. 2.8 A resolution crystal structure of human TRAIL, a cytokine with selective antitumor activity. Immunity 1999, 11 , 253-261.
5. Chen JJ, Chou CW, Chang YF, Chen CC.
Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. J Immunol 2008, 180 , 8030-8039.
6. Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem 1996, 65 , 801-847.
7. Drexler HC. Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci USA 1997, 94 , 855-860.
8. Ganten TM, Koschny R, Haas TL, Sykora J, Li- Weber M, Herzer K, Walczak H. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology 2005, 42 , 588-597.
9. Goldberg AL, Stein R, Adams J. New insights into proteasome function: from archaebacteria to drug development. Chem Biol 1995, 2 , 503-508.
10. Griffith TS, Anderson RD, Davidson BL, Williams
RD, Ratliff TL. Adenoviral-mediated transfer of the
TNF-related apoptosis-inducing ligand/Apo-2 ligand
gene induces tumor cell apoptosis. J Immunol 2000, 165 , 2886-2894.
11. Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Commun 2005, 331 , 718-725.
12. Hansson LO, Friedler A, Freund S, Rudiger S, Fersht AR. Two sequence motifs from HIF-1alpha bind to the DNA-binding site of p53. Proc Natl Acad Sci USA 2002, 99 , 10305-10309.
13. He Q, Huang Y, Sheikh MS. Proteasome inhibitor MG132 upregulates death receptor 5 and cooperates with Apo2L/TRAIL to induce apoptosis in Bax- proficient and -deficient cells. Oncogene 2004, 23 , 2554-2558.
14. Hougardy BM, Maduro JH, van der Zee AG, de Groot DJ, van den Heuvel FA, de Vries EG, de Jong S. Proteasome inhibitor MG132 sensitizes HPV- positive human cervical cancer cells to rhTRAIL- induced apoptosis. Int J Cancer 2006, 118 , 1892-1900.
15. Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O'Connell M, Kelley RF, Ashkenazi A, de Vos AM.
Triggering cell death: the crystal structure of Apo2L/
TRAIL in a complex with death receptor 5. Mol Cell 1999, 4 , 563-571.
16. Inoue T, Shiraki K, Fuke H, Yamanaka Y, Miyashita K, Yamaguchi Y, Yamamoto N, Ito K, Sugimoto K, Nakano T. Proteasome inhibition sensitizes hepato- cellular carcinoma cells to TRAIL by suppressing caspase inhibitors and AKT pathway. Anticancer Drugs 2006, 17 , 261-268.
17. Ishizawa J, Yoshida S, Oya M, Mizuno R, Shinojima T, Marumo K, Murai M. Inhibition of the ubiquitin- proteasome pathway activates stress kinases and induces apoptosis in renal cancer cells. Int J Oncol 2004, 25 , 697-702.
18. Kim K, Takimoto R, Dicker DT, Chen Y, Gazitt Y, El-Deiry WS. Enhanced TRAIL sensitivity by p53 overexpression in human cancer but not normal cell lines. Int J Oncol 2001, 18 , 241-247.
19. Kim OH, Lim JH, Woo KJ, Kim YH, Jin IN, Han ST, Park JW, Kwon TK. Influence of p53 and p21Waf1 expression on G2/M phase arrest of colorectal carcinoma HCT116 cells to proteasome inhibitors. Int J Oncol 2004, 24 , 935-941.
20. King RW, Deshaies RJ, Peters JM, Kirschner MW.
How proteolysis drives the cell cycle. Science 1996, 274 , 1652-1659.
21. Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med 2000, 6 , 513-519.
22. Lashinger LM, Zhu K, Williams SA, Shrader M, Dinney CP, McConkey DJ. Bortezomib abolishes tumor necrosis factor-related apoptosis-inducing ligand resistance via a p21-dependent mechanism in human bladder and prostate cancer cells. Cancer Res 2005, 65 , 4902-4908.
23. Lim ML, Lum MG, Hansen TM, Roucou X, Nagley P. On the release of cytochrome c from mitochondria during cell death signaling. J Biomed Sci 2002, 9 , 488- 24. 506. Ling YH, Liebes L, Ng B, Buckley M, Elliott PJ, Adams J, Jiang JD, Muggia FM, Perez-Soler R. PS- 341, a novel proteasome inhibitor, induces Bcl-2 phosphorylation and cleavage in association with G2- M phase arrest and apoptosis. Mol Cancer Ther 2002, 1 , 841-849.
25. Lu M, Dou QP, Kitson RP, Smith DM, Goldfarb RH. Differential effects of proteasome inhibitors on cell cycle and apoptotic pathways in human YT and Jurkat cells. J Cell Biochem 2006, 97 , 122-134.
26. Mlynarczuk I, Hoser G, Grzela T, Stoklosa T, Wójcik C, Malejczyk J, Jakóbisiak M. Augmented pro-apoptotic effects of TRAIL and proteasome inhibitor in human promonocytic leukemic U937 cells.
Anticancer Res 2001, 21 , 1237-1240.
27. Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K Jr, Huang P, Abbruzzese JL, McConkey DJ. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res 2005, 65 , 11658-11666.
28. Newmeyer DD, Ferguson-Miller S. Mitochondria:
releasing power for life and unleashing the machineries of death. Cell 2003, 112 , 481-490.
29. Pagano M. Cell cycle regulation by the ubiquitin pathway. FASEB J 1997, 11 , 1067-1075.
30. Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996, 271 , 12687- 12690.
31. Santer FR, Bacher N, Moser B, Morandell D,
Ressler S, Firth SM, Spoden GA, Sergi C, Baxter
RC, Jansen-Dürr P, Zwerschke W. Nuclear insulin-
like growth factor binding protein-3 induces apoptosis
and is targeted to ubiquitin/proteasome-dependent
proteolysis. Cancer Res 2006, 66 , 3024-3033.
32. Seol DW, Billiar TR. Cysteine 230 modulates tumor necrosis factor-related apoptosis-inducing ligand activity.
Cancer Res 2000, 60 , 3152-3154.
33. Voortman J, Resende TP, Abou El Hassan MA, Giaccone G, Kruyt FA. TRAIL therapy in non-small cell lung cancer cells: sensitization to death receptor- mediated apoptosis by proteasome inhibitor bortezomib.
Mol Cancer Ther 2007, 6 , 2103-2112.
34. Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand
invivo