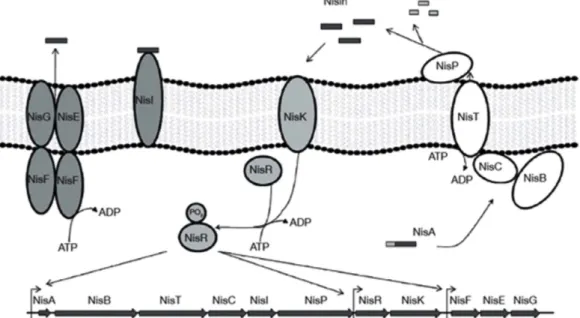
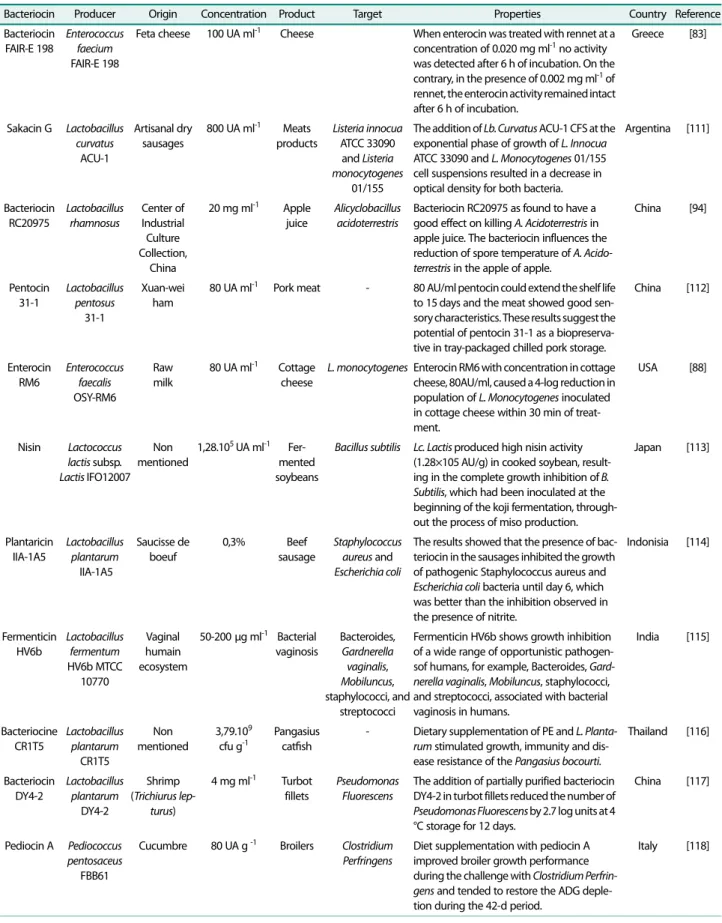
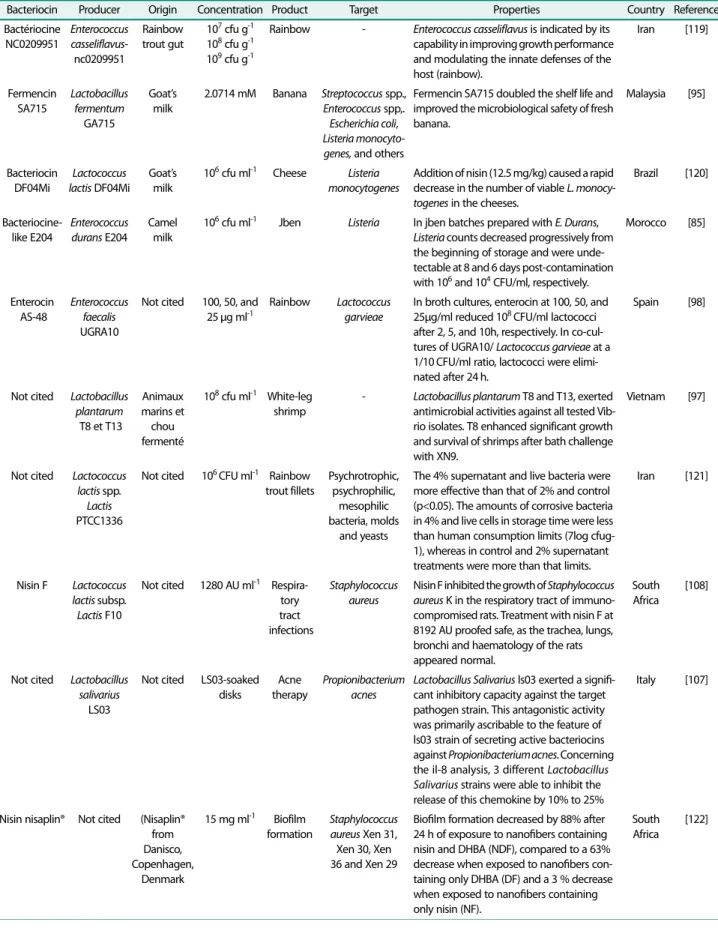
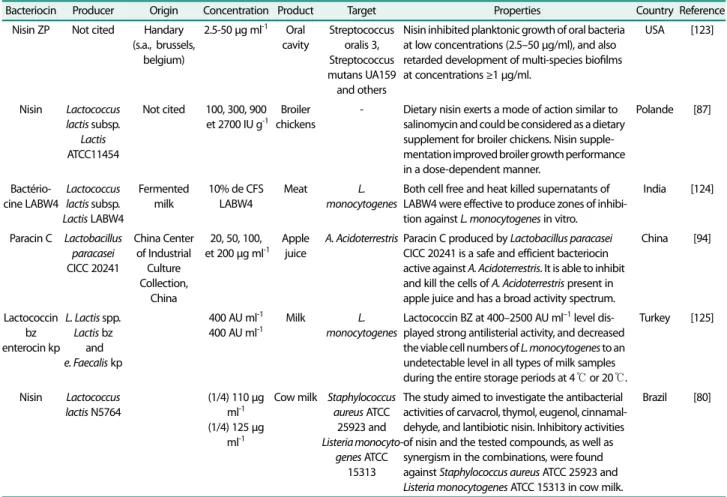
관련 문서
The obligate methanotrophic bacteria Methylococcus, Methylomonas, Methylosinus and related bacteria. Schleifer
Changes of lactic acid bacteria numbers and photodiode during fermentation mode and storage mode in S kimchi · · ·40..
In the third stage, strictly anaerobic methane fermentation, biogas was produced by methanogenic bacterium using organic acids including acetic acid.. Biogas consisted
- A novel lactic acid bacterium for the production of high purity l-lactic acid, Lactobacillus paracasei subspL. helveticus - Production of Lactic Acid from Water Hyacinth
The porous PCL scaffolds produced from the gas foaming and salt leaching and plasma surface treatment are suitable for potential applications in bone
Spoilage potential of psychrotrophic lactic acid bacteria (LAB) species: Leuconostoc gelidum subsp. gasicomitatum and Lactococcus piscium , on sweet bell
The purpose and necessity of this study was to investigate the effects of lactic acid fatigue and body oxidation through myofascial relaxation exercise using small tools
Probiotic properties of lactic acid bacteria isolated from mukeunji, a long-term ripened kimchi. Journal of Food
![Table 1. The main species of probiotics [32].](https://thumb-ap.123doks.com/thumbv2/123dokinfo/5440516.234665/3.892.118.783.178.530/table-the-main-species-of-probiotics.webp)