766
통신저자:박 상 진
전남 화순군 화순읍 일십리 160
전남대학교 의과대학 정형외과학교실, 화순전남대병원 관절센터 TEL: 061-379-7676ㆍFAX: 061-379-7681
E-mail: eksong@chonnam.ac.kr
Address reprint requests to Sang-Jin Park, M.D.
Department of Orthopaedic Surgery, Chonnam National University Hwasun Hospital, 160, Ilsim-ri, Hwasun-eup, Hwasun-gun, Jeonnam 519-809, Korea Tel: +82.61-379-7676, Fax: +82.61-379-7681
E-mail: eksong@chonnam.ac.kr
Gene Polymorphism Analysis Related to the Development of Deep Vein Thrombosis
Eun-Kyoo Song, M.D., Ph.D., Jong-Keun Seon, M.D., Sang-Jin Park, M.D., Young-Jin Kim, M.D., and Young-Hoon Park, M.D.
Investigation Performed at the Department of Orthopedic Surgery, Center for Joint Disease, Chonnam National University Hwasun Hospital, Jeonnam, Korea
심부 정맥 혈전증의 발생과 관련된 변형 유전자의 분석
송은규ㆍ선종근ㆍ박상진ㆍ김영진ㆍ박영훈
전남대학교 의과대학 정형외과학교실, 화순전남대병원 관절센터Purpose: To determine if the presence of thrombophilic genes affects the development of deep vein thrombosis after total knee arthroplasty (TKA).
Materials and Methods: One hundred and forty consecutive patients who underwent primary total knee arthroplasty were enrolled in this study. Preoperatively, all the patients were evaluated for general and hematological risk factors, as well as associated thrombophilic gene polymorphisms. Molecular genetic testing was carried out to detect the following thrombophilic gene polymorphisms: the factor V Leiden mutation, prothrombin promoter G20210A mutation, methylenetetrahydrofolate reductase (MTHFR) C677T, and the plasminogen activator inhibitor-1 (PAI-1) 4G/5G polymorphism.
Results: Deep vein thrombosis was observed in 48 (34%) patients. Regarding the genetic factors, no mutations in factor V Leiden or prothrombin promoter G20210A were detected in any patient regardless of whether they had DVT. The T677T and C677T genotypes of the MTHFR gene had no effect on the development of DVT compared with the C677C genotype. Regarding the PAI-1 gene, the presence of 4G/4G was not associated with an increased risk of DVT. In addition, there was a similar prevalence of the 4G allele in the DVT patients (77%) and controls (84%).
Conclusion: Thrombophilic gene polymorphisms do not appear to play an important role in the develop- ment of deep vein thrombosis. Therefore, screening tests for these genes will not be useful in determining the risk of deep vein thrombosis in our ethnic patients.
Key Words: Thrombophilic gene, Deep vein thrombosis, Total knee arthroplasty
INTRODUCTION
It is widely recognized that orthopedic surgery to the lower extremities is associated with an extra- ordinarily high incidence of postoperative deep vein thrombosis (DVT), which is the most common cause of morbidity and mortality after hip or knee arthroplasty16,18,25). In the absence of thrombopro-
phylaxis, studies from Western countries have re- ported a 40 to 84% incidence of venographically proven venous thrombo-embolism (VTE) after either total knee arthroplasty (TKA) or total hip arthroplasty (THA)10,25), and an approximately 10- 30% prevalence of pulmonary embolism (PE) in patients with DVT, with approximately 1-10% of
cases being fatal26). Even after effective thrombo- prophylaxis, DVT and PE develops in 10% to 20%
and 1% to 2% of patients after total joint re- placement, respectively22,33). Therefore, almost all patients undergoing total joint arthroplasty are treated prophylactically with mechanical or anti- coagulation therapy or both to prevent throm- boembolic events4,16,36). Few authors disagree that proximal DVT and PE are indications for anti- coagulation therapy16). However, it is unclear if thromboprophylaxis produces a good outcome.
Sound evidence suggests that chemical thrombo- prophylaxis reduces the venographic prevalence of VTE. However, there is little to suggest that this reduces the incidence of symptoms38). In addition, treatment with heparin is often associated with secondary complications. The incidence of DVT in the East Asian population after hip and knee arthroplasty is quite low13-18). Therefore, a deter- mination of the risk factors and selective chemical prophylaxis for patients with these risk factors would be useful. The aim of this study was to de- termine if the level of known thrombophilic gene polymorphisms is higher in patients who developed DVT after TKA than in a control group of patients who did not suffer any thromboembolic compli- cations after TKA. In addition, this study examined the ability of these thrombophilic gene molecular tests to determine the risk of the development of DVT in patients after TKA.
MATERIALS AND METHODS
One hundred and fifty consecutive patients, who received primary TKA between April 2006 and July 2006 at our hospital, were initially examined.
Patients, with degenerative osteoarthritis only, not rheumatoid arthritis, osteonecrosis and posttrau- matic arthritis, were included. Patients with a history of previous thromboembolic diseases were excluded because this study evaluated the new
development of DVT after TKA. Therefore, 10 patients (8 had other diagnoses, and 2 had a history of previous thromboembolic disease) were excluded. Overall, 140 patients were enrolled in this study cohort. All patients provided informed consent and the institutional review board ap- proved this prospective study. Of these, 35 and 105 patients underwent a one-stage bilateral TKA and unilateral TKA, respectively. All the TKAs in this study were performed using E-motion (B. Braun- Aesculap, Tuttlingen, Germany). There were 16 men and 124 women with a mean age 68.1 years (range, 49 to 85). All the patients were followed up for at least 3 months after surgery because throm- boembolic disease is the most common cause of death within 3 months after joint replacement35). No specific primary chemical prophylaxis against DVT was administered. All the patients were given elastic compressive stockings after surgery and were encouraged to perform an active range of motion exercises of the lower extremities from the second postoperative day. Not all patients with positive findings of DVT in computed tomography (CT) angiography were treated with anticoagu- lation therapy. Only those patients who had a symptomatic DVT were treated with therapeutic anticoagulation.
Preoperatively, all study subjects were evaluated for the risk factors of general, hematological, and thrombophilic gene polymorphisms. The general risk factors such as age, gender, high body mass index (BMI, kg/m2), smoking, diabetes, hyperten- sion, presence of varicose veins, history of mali- gnancy, underlying heart disease, bilateral TKA, general anesthesia and long tourniquet time, were also evaluated. Blood samples were obtained from all patients preoperatively to evaluate the hema- tological abnormalities and the presence of throm- bophilic gene polymorphisms. The level of factors VIII, IX, and XI, fibrinogen, antithrombin III, pro-
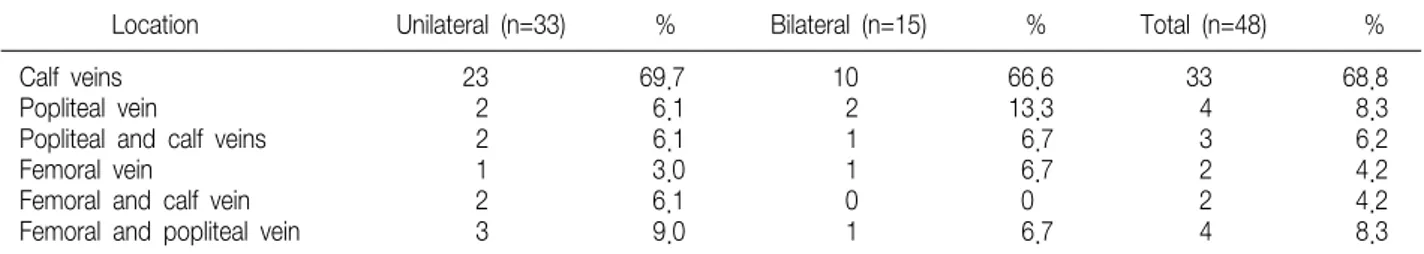
Table 1. The Locations of the Thrombi in 48 Patients with Deep Vein Thrombosis after Total Knee Arthroplasty
Location Unilateral (n=33) % Bilateral (n=15) % Total (n=48) %
Calf veins 23 69.7 10 66.6 33 68.8
Popliteal vein 2 6.1 2 13.3 4 8.3
Popliteal and calf veins 2 6.1 1 6.7 3 6.2
Femoral vein 1 3.0 1 6.7 2 4.2
Femoral and calf vein 2 6.1 0 0 2 4.2
Femoral and popliteal vein 3 9.0 1 6.7 4 8.3
tein C and S, and hemoglobin as well as the platelet counts were examined as hypercoagulable and hypofibrinolytic factors. Molecular genetic testing was carried out to detect the following throm- bophilic gene polymorphisms that have been asso- ciated with DVT: the factor V Leiden mutation, prothrombin promoter G20210A mutation, methy- lenetetrahydrofolate reductase (MTHFR) C677T, and the plasminogen activator inhibitor-1 (PAI-1) 4G/5G polymorphism. Molecular analyses of the above-listed genes were performed by single poly- merase chain reactions using the patient's genomic DNA isolated from the peripheral blood leuko- cytes1,3,28).
For a diagnosis of DVT, images of 64 channel multidetector CT venography (LightSpeed VCT, GE Healthcare, Waukesha, Wisconsin) were obtained from all patients seven days after surgery or at the time when the symptoms of DVT first appeared.
UltravistⓇ (Schering, Berlin, Germany) was used as the contrast medium. A single expert radiologist confirmed the positive findings of a filling defect in a vessel or defects surrounded by a narrow rim of contrast material. Ultrasonography was also performed to rule out DVT when CT venography produced questionable findings around an implant.
The statistical difference was analyzed using a Student T-test for age, obesity and tourniquet time, and a chi-square test for any other general risk factor. The correlation between the develop- ment of DVT and hematologic risk factor or thrombophilic gene polymorphism was examined
using a chi-square test with the odds ratio and a confidence interval of 95%.
RESULTS
Of the 140 consecutive patients examined, DVT was diagnosed in 48 (34%) patients (33 and 15 patients in the unilateral and bilateral TKA group, respectively). Table 1 shows the locations of the thrombi. No patient suffered from a pulmonary embolism. In the unilateral TKA group, most patients (23, 70.3%) had thrombi in the calf veins, which extended proximally into the popliteal and/
or femoral vein in 10 (29.7%) patients. In the bilateral TKA group, most patients (10, 66.7%) had thrombi in the calf veins, which extended proxi- mally in 5 (33.3%) patients. Of these 48 patients, only 5 (10.4%) showed symptoms of DVT, which consisted of 2 unilateral TKA and 3 bilateral TKA patients. All the patients complained calf pain.
There was calf tenderness, swelling and positive Homan's signs (increased resistance or pain to ankle dorsiflexion). Anticoagulant treatment using intravenous heaprinization for acrivated partial thromboplastin time (aPTT) was used at approxi- mately twice the control value. Hepainization was changed to oral wafarin to maintain a prothrombin time (PT) of INR 2.0 to 3.0, which was continued for 3 months for the bilateral cases and 6 weeks for the unilateral cases. All the patients were not severe and recovered after anticoagulation treat- ment without complications.
Regarding the general risk factors, only obesity
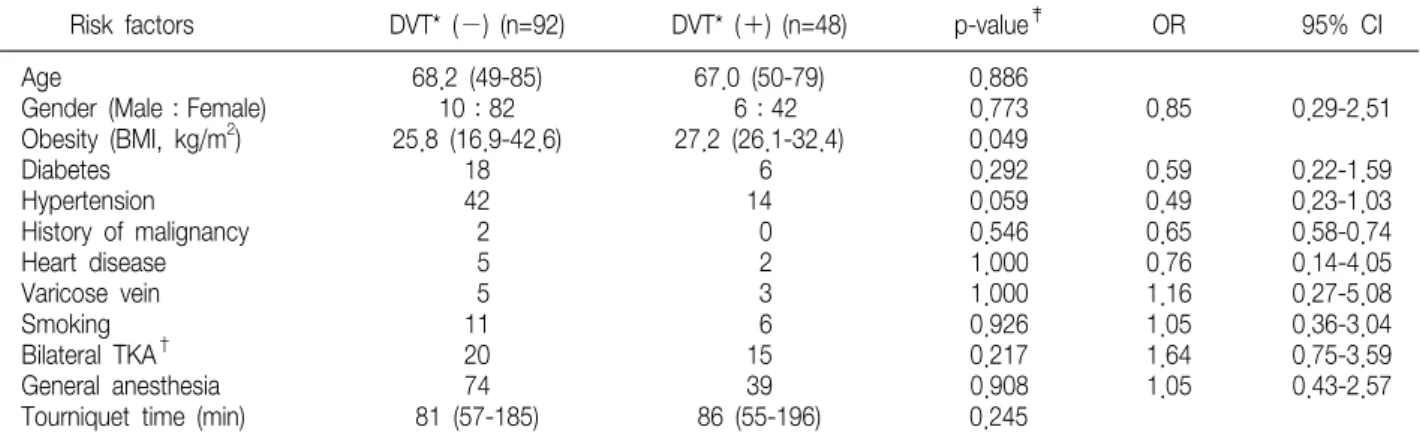
Table 2. General Risk Factors of Venous Thromboembolism after Total Knee Arthroplasty
Risk factors DVT* (−) (n=92) DVT* (+) (n=48) p-value‡ OR 95% CI
Age 68.2 (49-85) 67.0 (50-79) 0.886
Gender (Male:Female) 10:82 6:42 0.773 0.85 0.29-2.51
Obesity (BMI, kg/m2) 25.8 (16.9-42.6) 27.2 (26.1-32.4) 0.049
Diabetes 18 6 0.292 0.59 0.22-1.59
Hypertension 42 14 0.059 0.49 0.23-1.03
History of malignancy 2 0 0.546 0.65 0.58-0.74
Heart disease 5 2 1.000 0.76 0.14-4.05
Varicose vein 5 3 1.000 1.16 0.27-5.08
Smoking 11 6 0.926 1.05 0.36-3.04
Bilateral TKA† 20 15 0.217 1.64 0.75-3.59
General anesthesia 74 39 0.908 1.05 0.43-2.57
Tourniquet time (min) 81 (57-185) 86 (55-196) 0.245
*DVT, deep vein thrombosis; †TKA, total knee arthroplasty; ‡Student t-test for continuous variables and Chi-square test or Fisher's exact test for discrete variables.
Table 3. Comparison of the Hematological Risk Factors of Deep Vein Thrombosis after Total Knee Arthroplasty
DVT* DVT*
Risk factors (−) (+) OR† 95% CI‡ (n=92) (n=48)
Factor VIII>150% 17 10 1.16 0.49-2.78
Factor IX>150% 11 6 1.05 0.36-3.04
Factor XI>140% 10 9 1.89 0.71-5.03
Fibrinogen>400 mg/dl 30 17 1.13 0.54-2.36
Antithrombin III<70% 0 0 − −
Protein C<65% 0 0 − −
Protein S<65% 0 0 − −
Hemoglobin>18 g/dl 0 0 − −
Platelet>450×103/mm2 9 4 0.75 0.22-2.52
*DVT, deep vein thrombosis; †OR, odds ratio; ‡CI, confidence interval.
(BMI) was significantly associated with an in- creased incidence of DVT (p=0.049) (Table 2). There were no hematological abnormalities (elevated levels of factor VIII (>150%), IX (>150%), and XI (>140%), plasma fibrinogen (>400 mg/dl), and platelet counts (>450×103/mm2)) found to be associated with an increased risk of DVT (Table 3).
In addition, no DVT positive or DVT negative patient showed evidence of any abnormality upon screening with antithrombin III, protein C and S, or according to the hemoglobin level (Table 3).
Regarding the genetic factors, no mutations in factor V Leiden and prothrombin promoter G20210A were detected in any patient regardless of whether they had developed DVT. The T677T and C677T genotypes of the MTHFR gene had no effect on the development of DVT compared with the C677C genotype (Table 4). Regarding the PAI-1 gene, the presence of 4G/4G was not associated with an increased risk of DVT (OR, 0.58; 95% CI, 0.22- 1.48) (Table 4). In addition, there was a similar prevalence of the 4G allele in the DVT patients (77%) and DVT negative patients (84%).
DISCUSSION
If there is a functional and genetic test that can
identify the risk of thrombophilia and hypofibri- nolysis, only patients with a demonstrated high risk of thromboembolic disease will be given post- operative anticoagulation treatments, which would optimize the risk-benefit ratio for prophylaxis against thromboembolic disease after TKA39). One article27) documented a complication rate of up to 45% due to chemoprophylaxis, such as hema- toma, wound dehiscence, and gastrointestinal and cerebral bleeding. Although almost all throm- boembolic and iatrogenic complications in patients with a thrombosis will eventually resolve, the
Table 4. Distribution of Thrombophilic Gene Polymorphisms in Patients who Underwent Total Knee Arthroplasty with and without Deep Vein Thrombosis
DVT* (−) DVT* (+)
OR 95% CI
(n=92) (%) (n=48) (%)
Factor V Leiden mutation 0 0 − −
Prothrombin promoter 0 0 − −
G20210A mutation
Methylenetetrahydrofolate reductase mutation
C677C 29 (32) 16 (33) 1.0*
C677T 46 (50) 23 (48) 0.91 0.41-2.00
T677T 17 (18) 9 (19) 0.96 0.35-2.64
Plasminogen activator-1 mutation
5G/5G 15 (16) 11 (23) 1.0†
5G/4G 32 (35) 18 (38) 0.77 0.29-2.02
4G/4G 45 (49) 19 (39) 0.58 0.22-1.48
*Reference category C677C; †Reference category 5G/5G.
duration of hospitalization is prolonged, and some patients will have permanent disability as a result.
Warwick and Whitehouse suggested that chemical prophylaxis does not reduce the frequency of sym- ptomatic thromboembolic events (10.1% vs 10.5%
with and without chemical prophylaxis, respec- tively). Moreover, hematoma or wound dehiscence was more common in those with chemical pro- phylaxis38).
In addition, in three series where chemical pro- phylaxis had not been used, Khaw et al13) reported only one fatal PE out of 499 patients (0.2%), Kim15) reported none in 244 (0%), and Warwick and Whitehouse38) reported one out of 1000 patients (0.1%), which is consistent with our rate of 0%. In addition, East Asians have a lower incidence of DVT (even without thromboprophylaxis, ranging 13% to 43%) after TKA than Caucasians16,18). Con- sidering the low incidence of thrombosis after TKA, it is believed that the routine screening for hema- tological and genetic abnormalities as well as routine chemical thromboprophylaxis is unne- cessary in East Asians.
Generally, the incidence of DVT after TKA in- creases with age, female gender, obesity, diabetes, hypertension, a history of malignancy, underlying
heart disease, varicose veins, smoking, bilateral rather than unilateral TKA, a prolonged operation time, prolonged immobilization, general anesthe- sia, cemented type prosthesis, increased pro- thrombotic hematologic profiles (elevated plasma levels of factors VIII, IX, and XI, as well as increased levels of fibrinogen, hemoglobin, and platelets, and the presence of antiphospholipid antibody and lupus anticoagulant antibody), de- creased antithrombotic hematological profiles (a deficiency of the antithrombin III, and of protein C and S), and the presence of antiphospholipid antibody11,19,20,21,24,30,37)
. However, in this study, no general or hematological risk factors except for obesity (BMI) were associated with an increased risk of DVT in patients undergoing primary TKA.
Several inherited thrombophilic gene polymor- phisms have recently been identified as increasing the risk of thrombosis. A deficiency in the follow- ing is each associated with hypercoagulable states, and increase the risk of thromboembolism 3 to 80 fold: natural anticoagulant proteins, antithrombin III, protein C and protein S; resistance to activated protein C; the presence of factor V Leiden, pro- thrombin promoter G20210A mutation, MTHFR T677T mutation, PAI-1 4G/4G polymorphism, or
platelet glycoprotein IIb/IIIa A1/A2 or A2/A2 poly- morphism; and elevated plasma levels of fibrino- gen or homocysteine11,21,25,34,39)
.
A factor V Leiden mutation is one of the most common genetic thrombophilic defects with an overall prevalence of approximately 5% of carriers among Caucasians, even though there are regional differences due to founder effects. This mutation was found in 20% of venous thrombosis patients and in up to 50% of patients with thrombophilia, and is known to increase the risk of thrombosis by approximately 3 to 80 fold21,31). Like a Factor V Leiden mutation, a prothrombin 20210A mutation is observed only in Caucasians with a prevalence of 2% to 3%21,31). This mutation increases the risk of thrombosis approximately 3 fold, which is me- diated through elevated prothrombin levels2,5). A variant in the gene for MTHFR, which plays a role in elevating the homocysteine levels, is also asso- ciated with an increased risk of thrombosis. Its prevalence among Caucasians is approximately 10%. Meta-analysis of 53 studies (n=8364 cases) revealed MTHFR T677T homozygosity to be asso- ciated with a 20% increased risk of venous thrombosis compared with MTHFR C677C homozy- gosity7,8,31). PAI-1 is a major inhibitor of the tissue type plasminogen activator (t-PA). Reduced fibri- nolytic ability due to increased plasma PAI-1 levels has been suggested to play an important role in the pathogenesis of disorders associated with throm- bosis. Moreover, the PAI-1 levels are affected by PAI-1 gene polymorphisms. Individuals homozy- gous for the 4G allele have increased plasma PAI-1 concentrations compared with those with the 5G allele, and the presence of 4G/4G homozygosity is associated with a 3 to 20 fold increased risk of thrombosis6,21,31).
In this study, there were no factor V Leiden and prothrombin 20210A mutations observed in the DVT positive and DVT negative patients after TKA.
These results are similar to those reported in other Chinese23), Japanese9), and Korean14) studies. In addition, while factor V Leiden accounts for 7% of nonsurgical venous thrombosis29), these observa- tions are consistent with those in three recent prospective studies of orthopedic patients, in which factor V Leiden was found to be noncontri- butory32,39,40). There was a relatively higher pre- valence of MTHFR T677T homozygosity in the two study groups (19% in DVT positive group and 18%
in DVT negative group). However, no significant correlation was found between the presence of MTHFR T677T homozygosity and the development of DVT. This result is also similar with that of another East Asian study23). Regarding PAI-1 gene polymorphisms, the study subjects showed a high prevalence of the 4G allele but there was no clinical correlation between its presence and the develop- ment of DVT.
CONCLUSION
Although only four molecular genetic tests (factor V Leiden, prothrombin promoter G20210A, MTHFR C677T and PAI-1 4G/5G mutations) were used in this study, these thrombophilic gene poly- morphisms do not appear to be associated with the development of the DVT after TKA. Therefore, these molecular genetic tests would not be useful for determining the risk of deep vein thrombosis in our ethnic patients.
REFERENCES
1. Balta G, Altay C, Gurgey A: PAI-1 gene 4G/5G genotype:
a risk factor for thrombosis in vessel of internal organs. Am J Hematol, 71: 89-93, 2002.
2. Bank I, Libourel EJ, Middeldorp S, et al: Prothrombin 20210A mutation: a mild risk factor for venous thromboem- bolism but not for arterial thrombotic disease and pregnancy- related complications in a family study. Arch Intern Med, 164:
1932-1937, 2004.
3. Bertina RM, Koeleman BPC, Koster T, et al: Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature, 369: 64-67, 1994.
4. Ceelie H, Spaargaren-van Riel CC, Bertina RM, Vos HL:
G20210A is a functional mutation in the prothrombin gene;
effect on protein levels and 3'-end formation. J Thromb Haemost, 2: 119-127, 2004.
5. Dawson SJ, Wiman B, Hamsten A, Green F, Humphries S, Henney AM: The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J Biol Chem, 268: 10739-10745, 1993.
6. de Groot PG, Lutters B, Derksen RH, Lisman T, Meijers JC, Rosendaal FR: Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost, 3:
1993-1997, 2005.
7. Den Heijer M, Lewington S, Clarke R: Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost, 3: 292- 299, 2005.
8. Frosst P, Blom HJ, Milos R, et al: A candidate genetic risk factor for vascular disease: a common mutation in methylene- tetrahydrofolate reductase. Nat Genet, 10: 111-113, 1995.
9. Fujimura H, Kambayashi J, Monden M, Kato H, Miyata T: Coagulation factor V Leiden mutation may have a racial background. Thromb Haemost, 74: 1381-1382, 1995.
10. Geerts WH, Heit JA, Clagett GP, et al: Prevention of venous thromboembolism. Chest, 119(Suppl 1): S132-S175, 2001.
11. Ginsberg JS, Wells PS, Brill-Edwards P, et al: Anti- phospholipid antibodies and venous thromboembolism. Blood, 86: 3685-3691, 1995.
12. Heit JA, O'Fallon WM, Petterson TM, et al: Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med, 162:
1245-1248, 2002.
13. Khaw FM, Moran CG, Pinder IM, Smith SR: The incidence of fatal pulmonary embolism after knee replacement with no prophylactic anticoagulation. J Bone Joint Surg Br, 75:
940-941, 1993.
14. Kim TW, Kim WK, Lee JH, et al: Low prevalence of activated protein C resistance and coagulation factor V Arg506 to Gln mutation among Korean patients with deep vein thrombosis. J Korean Med Sci, 13: 587-590, 1998.
15. Kim YH: The incidence of deep vein thrombosis after cementless and cemented knee replacement. J Bone Joint Surg Br, 72: 779-783, 1990.
16. Kim YH, Kim JS: Incidence and natural history of deep-vein thrombosis after total knee arthroplasty. A prospective, rando- mized study. J Bone Joint Surg Br, 84: 566-570, 2002.
17. Kim YH, Suh JS: Low incidence of deep-vein thrombosis after cementless total hip replacement. J Bone Joint Surg Am, 70:
878-882, 1988.
18. Ko PS, Chan WF, Siu TH, Khoo J, Wu WC, Lam JJ: Deep vein thrombosis after total hip or knee arthroplasty in a
"low-risk" Chinese population. J Arthroplasty, 18: 174-179, 2003.
19. Kohler HP, Grant PJ: Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med, 342: 1792-1801, 2000.
20. Koster T, Rosendaal FR, Briët E, et al: Protein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (Leiden Throm- bophilia Study). Blood, 85: 2756-2761, 1995.
21. Kyrle PA, Minar E, Hirschl M, et al: High plasma levels of factor VIII and risk of recurrent venous thromboembolism.
N Engl J Med, 343: 457-462, 2000.
22. Lane DA, Grant PJ: Role of hemostatic gene polymorphisms in venous and arterial thrombotic disease. Blood, 95: 1517- 1532, 2000.
23. Leyvrza PF, Richard J, Bachmann F, et al: Adjusted versus fixed-dose subcutaneous heparin in the prevention of deep-vein thrombosis after total hip replacement. N Engl J Med, 309:
954-958, 1983.
24. Lu Y, Zhao Y, Liu G, et al: Factor V gene G1691A muta- tion, prothrombin gene G20210A mutation, and MTHFR gene C677T mutation are not risk factors for pulmonary throm- boembolism in Chinese population. Thromb Res, 106: 7-12, 2002.
25. Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM,
Rosendaal FR: High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med, 342: 696-701, 2000.
26. Mont MA, Jones LC, Rajadhyaksha AD, et al: Risk factors for pulmonary emboli after total hip or knee arthro- plasty. Clin Orthop Relat Res, 422: 154-163, 2004.
27. Moser KM: Pulmonary embolism. Am Rev Respir Dis, 115:
829-852, 1977.
28. Oger E: Incidence of venous thromboembolism: a community- based study in western France. EPI-GETBP study group.
Group d'Etude de la thrombose de bretagne occidentale.
Thromb Haemost, 83: 657-660, 2000.
29. Patterson BM, Marchand R, Ranawat C: Complications of heparin therapy after total joint arthroplasty. J Bone Joint Surg Am, 71: 1130-1134, 1989.
30. Poort SR, Rosendaal FR, Reitsma PH, Bertina RM: A common genetic variation in the 3'-untranslated region of the prothrombin gene is associated with elevated plasma proth- rombin levels and an increase in venous thrombosis. Blood, 88:
3698-3703, 1996.
31. Rodeghiero F, Tosetto A: Activated protein C resistance and factor V Leiden mutation are independent risk factors for venous thromboembolism. Ann Intern Med, 130: 643-650, 1999.
32. Rosendaal FR: Risk factors for venous thrombosis: prevalence, risk and interaction. Semin Hematol, 34: 171-187, 1997.
33. Rosendaal FR: Venous thrombosis: the role of genes, envi- ronment, and behavior. Hematology Am Soc Hematol Educ
Program, 1-12, 2005.
34. Ryan DH, Crowther MA, Ginsberg JS, Francis CW:
Relation of factor V Leiden genotype to risk for acute deep venous thrombosis after joint replacement surgery. Ann Intern Med, 128: 270-276, 1998.
35. Sharrock NE, Brien WW, Salvati EA, Mineo R, Garvin K, Sculco TP: The effect of intravenous fixed-dose heparin during total hip arthroplasty on the incidence of deep-vein thrombosis. A randomized, double-blind trial in patients operated on with epidural anesthesia and controlled hypo- tension. J Bone Joint Surg Am, 72: 1456-1461, 1990.
36. Simioni P, Prandoni P, Zanon E, et al: Deep venous thrombosis and lupus anticoagulant. A case-control study.
Thromb Haemost 76: 187-189, 1996.
37. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB: Elevated C-reactive protein levels in overweight and obese adults. JAMA, 282: 2131-2135, 1999.
38. Warwick DJ, Whitehouse S: Symptomatic venous throm- boembolism after total knee replacement. J Bone Joint Surg Br, 79: 780-786, 1997.
39. Westrich GH, Weksler BB, Glueck CJ, Blumenthal BF, Salvati EA: Correlation of thrombophilia and hypofibrinolysis with pulmonary embolism following total hip arthroplasty: an analysis of genetic factors. J Bone Joint Surg Am, 84: 2161- 2167, 2002.
40. Woolson ST, Zehnder JL, Maloney WJ: Factor V Leiden and the risk of proximal venous thrombosis after total hip arthroplasty. J Arthroplasty, 13: 207-210, 1998.