13
Reference Range of HE4 in Healthy Women: Analytical Performance and Correlation with CA125
Kwang-Sook Woo, Jae-Lim Choi, Bo-Ram Kim, Ji-Eun Kim, and Jin-Yeong Han Departments of Laboratory Medicine, Dong-A University College of Medicine, Busan, Korea
건강한 성인 여성에서 HF4의 참고치: 분석적 수행능 및 CA125 와의 상관성 평가
우광숙․최재림․김보람․김지은․한진영 동아대학교 의과대학 진단검사의학교실
접수일: 11 / 12 / 2 최종재심접수일: 12 / 5 / 8 게재승인일: 12 / 5 / 31 교신저자:한진영
우)602-715 부산광역시 서구 동대 신동 3가 1번지, 동아대학교병원 진단검사의학과
전화 : 051)240-5323 FAX : 051)255-9366 E-mail : jyhan@dau.ac.kr
Background: Laboratory diagnosis for ovarian cancer is mostly based on the quantitative determination of CA125. Over the past years, a number of additional markers for ovarian cancer have been proposed and studied. Human epididymis protein 4 (HE4) has accordingly emerged as a new biomarker for the detection of ovarian cancer. To evaluate the new automated HE4 assay, we studied analytical performance, and established reference ranges.
Methods: We evaluated precision performances and linearity of the HE4 assay. We also evaluated reference ranges for HE4 and CA125 according to age. Lastly, we investigated the correlation between HE4 and CA125.
Results: The precision study showed excellent results for both high and low control. The 95% upper reference ranges for HE4 and CA125 levels were 81.0 pmol/L (90% confidence interval [CI], 63.0-103.1) and 28.6 U/mL (90% CI, 25.4-36.4), respectively. There was no correlation between HE4 and CA125 (r = -0.002, P = 0.9793) in healthy women. Reference ranges of HE4 tended to be slightly higher for the older groups as compared to the younger groups. CA125 were considerably decreased in the oldest age group (ages 70 to 79).
Conclusions: The new automated HE4 assay showed good analytical performance, age-related variable results and no correlation with CA125. Though further studies for clinical and diagnostic effectiveness of HE4 assay in screening and diagnosing ovarian cancer are needed for routine use of HE4, HE4 in combination with CA125 is likely to be more useful diagnostically than CA125 alone.
Key Words: HE4, CA125, Reference values
INTRODUCTION
Ovarian cancer is the fifth leading cause of cancer-related mortalities in women in the United States and the third ranking cause of death from oncologic diseases in the European Union [1,2]. In Korea, it is the second commonest gynecological cancer and mortality rates for ovarian cancer have
been increasing [3-5]. However, if detected in its early stages, ovarian cancer has a good prognosis [6-8]. Therefore, early detection is one of the most important factors associated with improving prognosis. Until now, CA125 has been the most widely used serum biomarker to predict ovarian cancer. However, up to 20% of ovarian cancers do not express CA125 [9], and CA125 can be elevated
J Lab Med Qual Assur 2012 ; 34:13-8
in many benign conditions. The sensitivity, specificity, and positive predictive value of CA125 are relatively low for population screening of early stage ovarian cancer [10,11]. Therefore, over the past years, a number of additional markers for ovarian cancer have been proposed and studied to improve the diagnostic performance. Among these, human epididymis protein 4 (HE4), has emerged as a new biomarker for the detection of ovarian cancer with relatively high sensitivity and specificity [12].
In a recent study, HE4 as a single marker or combined with CA125 showed the highest sensitivity among other combinations, especially in early stage ovarian cancers [13].
Nevertheless, there are few reports about reference ranges of HE4 established with a large healthy population, and HE4 levels in healthy women have been reported to increase with age [14, 15]. HE4 is not yet a suitable biomarker for ovarian cancer screening as a substitute for CA125.
Further, almost all of the previous studies with HE4 utilized enzyme-linked immunosorbent assays (ELISA) with lower analytical sensitivity and precision performance than automated assays utilizing the chemiluminescent immunoassay (CLIA) method.
In this study, we aimed to evaluate a new automated HE4 assay. We assessed performance of the assay and established a reference range for HE4, especially according to age, in a healthy population and compared this to a reference range that we obtained for CA125. Further, we investigated the possible correlation between HE4 and CA125.
MATERIALS AND METHODS
This study included 200 healthy women, who came to Dong-A University Hospital between September 2010 and March 2012. The healthy population consisted of women who visited the hospital for periodic medical checkups and were found to have no specific clinical or laboratory problems. Individuals with non-gynecologic diseases or gynecologic diseases were excluded from this study. Blood samples were obtained from the patients in this group of healthy individuals. The sera were separated immediately after arrival and
tested for biomarkers in a periodic medical checkup, which included CA125. Thereafter, all residual samples were stored at -70℃ until assayed for HE4. HE4 and CA125 were measured by a chemiluminescent microparticle immunoassay (CMIA) using Abbott Architect (Abbott Laboratories, Abbott Park, IL, USA). Assay precision (coefficient of variation, CV) and linearity of the HE4 assay were assessed. Assay precision of HE4 test was evaluated according to CLSI protocol EP5-A2 [16]. We tested the low and high control materials simultaneously.
We measured the HE4 concentration twice daily for five consecutive days. Tests for validating the linearity range of the HE4 assay were performed based on the CLSI protocol EP6A, samples with five levels were prepared by mixing high and low levels of pooled patients’ sera, and were tested in duplicate [17]. Reference ranges of HE4 and CA125 were also evaluated. In particular, we investigated the influence of age on HE4 and CA125, and evaluated reference ranges according to age. We divided the study population into 6 subgroups of ages 20 to 29 (N = 19), 30 to 39 (N = 32), 40 to 49 (N = 55), 50 to 59 (N = 39), 60 to 69 (N = 37), and 70 to 79 (N = 18), and the upper reference limits of each group were calculated. Because our study group is not in a normal distribution, we calculated the upper reference limits with a non-parametric method. The entire statistical analysis was performed using MedCalc version 9.3 (MedCalc Software, Mariakerke, Belgium). Levene’s test for equality of variances was performed to define the distributions of markers’ levels in the healthy population. Multiple comparisons between the age groups were performed using the one-way analysis of variance (ANOVA). Data were also analyzed with Analyse-it (Analyse-it Software Ltd., Leeds, UK).P values of less than 0.05 were regarded as statistically significant.
RESULTS
The characteristics of the study group are presented in Table 1. The median HE4 and CA125 levels of healthy individuals were 33.9 pmol/L and 12.2 U/mL. Distribution of values of the assay results and the 95th percentile upper reference limits of HE4 and CA125 are illustrated in Fig. 1.
Table 1. HE4 and CA125 concentrations in the study group*
Group (N=200) Age (years) HE4 (pmol/L) CA125 (U/mL)
Healthy individuals 49.0 (20-79) 33.9 (15.4-132.5) 12.2 (3.9-173.2)
*Data are given as median (range).
Table 2. Reference ranges of HE4 and CA125 according to age group
Age* (N) Upper reference limits†
HE4 (pmol/L) CA125 (U/mL)
20-29 (19) 67.7 25.4
30-39 (32) 54.7 61.5
40-49 (55) 42.9 27.9
50-59 (39) 56.7 105.8
60-69 (37) 82.2 17.8
70-79 (18) 132.5 21.6
P-value‡ < 0.001 0.002
*Data are given as range (number);†Data are given as upper reference limits with a non-parametric method;‡P values were calculated by comparison of each subgroup.
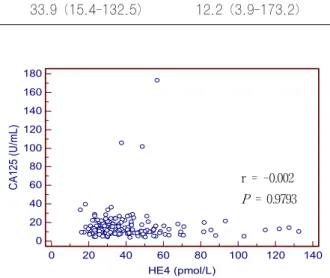
Fig. 1. Distributions of the assayed values and the 95th percentile upper reference limits of HE4 and CA125.
0 20 40 60 80 100 120 140
180 160 140 120 100 80 60 40 20 0
HE4 (pmol/L)
CA125 (U/mL)
r = -0.002 P = 0.9793
Fig. 3. Comparison of HE4 and CA125 assays. There was no correlation between HE4 and CA125.
y = 0.9612x - 1.9577 R2= 0.9984
Fig. 2. Linearity analysis of HE4 assay.
The 95% upper reference ranges for HE4 and CA125 levels were 81.0 pmol/L (90% confidence interval [CI], 63.0-103.1) and 28.6 U/mL (90% CI, 25.4- 36.4), respectively. Table 2 shows reference ranges of HE4 according to age group in healthy individuals. HE4 tended to be slightly higher for the older groups and CA125 was considerably decreased in the oldest age group (70-79). The result of the precision study showed that the within-run and between-run precisions (CV) were excellent with all less than 5%. Between-day precision was 3.8% with low control and 4.7% with high control and total precision was 4.5% (low control) and 4.7% (high
control) as well. The HE4 assay was shown to be linear for comparing measured values with expected values. The linearity could be expressed by the following equation: y =0.9612x - 1.9577, R2= 0.9984 (Fig. 2). There was no correlation between HE4 and CA125 (r =-0.002, P=0.9793) in our study population (Fig. 3).
DISCUSSION
In Korea, approximately 2,000 new cases of ovarian cancer are diagnosed annually and the prevalence of ovarian cancer is continuing to increase. Early stage ovarian cancer has an excellent prognosis if treated, but 70% of patients are diagnosed in advanced stages, which are associated with a poor survival rate of only 10-30%
[18]. Given the limitations of treatment for advanced ovarian cancer and the success of treatment for early stage disease, a screening test is very valuable. Until now, CA125 has been widely used for predicting ovarian cancer, although many studies have reported its low sensitivity and specificity for ovarian cancer [9-11]. Accordingly, many other markers such as mesothelin, CA72-4, inhibin, kallikreins, and osteopontin, have been investigated to complement CA125 and to improve its sensitivity for early detection of ovarian cancer [19-22]. Among these, HE4 is one of the most promising markers for improving the sensitivity and specificity [12]. Many studies have reported that as a single marker, HE4 had the highest sensitivity for detecting ovarian cancer especially early stage disease [13,15,19,23,24]. Furthermore, recent studies showed that HE4 or CA125, when used alone as a diagnostic tool had strengths and weaknesses [14,25,26]. Combining CA125 and HE4 might be more advantageous than either one alone [27-29]. One study showed 58% sensitivity and 98.5% specificity in predicting ovarian cancer using HE4 and CA125 [28]. A diagnostic algorithm using both HE4 and CA125 was made in another study that successfully categorized patients into high and low risk groups with about 94% of epithelial ovarian cancer correctly classified as high risk [29].
From these data, although we recognized the need for more than one marker, e.g., both HE4 and CA125 to predict ovarian cancer, there are few
published studies for reference ranges of HE4 in healthy individuals. In this study, we evaluated reference ranges of HE4 and CA125 according to age. Reference ranges of HE4 tended to be slightly higher for the older age groups, while those of CA125 were considerably decreased in the oldest age group. This result is in agreement with a more recent study, which evaluated the reference range of HE4 and CA125 according to age group for a homogeneous population consisting of healthy Asian female hospital workers [14]. In that study, HE4 consequently showed better diagnostic performance than CA125 when using cutoffs specific to the age group and HE4 seemed to be a more useful biomarker with less age-related variation and greater sensitivity for detecting ovarian cancer than CA125. In our study, although HE4 showed good analytical performance in agreements with previous studies, CA125 was less variable. Because we included only healthy individuals, we could not evaluate the diagnostic performance of HE4 and CA 125. Many previous studies reported that HE4 alone was a more useful marker than CA125 in various circumstances including detection of ovarian cancer from various gynecologic and non-gynecologic diseases, comparison of nonmalignant disease versus early stage ovarian cancer, and combined CA125 and HE4 showed the highest sensitivity in detecting ovarian cancer [13,15,19,23,24,27-29]. In a few studies, CA125 was more useful in postmenopausal women than in premenopausal women, because CA125 levels in healthy postmenopausal women have been found to be significantly lower than those in premenopausal women [26,30]. Therefore, lower CA125 concentrations in older age groups might be the result of menopausal status though we did not collect data about the menopausal status of the individuals.
The HE4 assay used in this study showed good precision performance with less than 5% CV in all assessed samples in contrast to ELISA that is generally known to have lower precision and analytical sensitivity than CMIA or CLIA methods.
Recent analytical studies with the automated HE4 assay also showed good precision performance with less than 5% CV on the low, medium and high-level controls [14,31]. We showed that there was no correlation between HE4 and CA125 and there were
some discordant results especially in the oldest age group with significantly low CA125. This means that each marker is elevated differently under different conditions, and these findings also support the necessity of combining the two markers. In addition, some studies that showed the difference between elevation patterns of both markers in benign conditions would also emphasize the need for multiple markers in combination [14,25-29].
There are some limitations in our study. The smaller population in our study, especially in the older age group, the difference of sample size between the age groups and selection of a reference group may influence the results. Ethnic differences and method differences for evaluating HE4 and CA125 might also affect our result. HE4 and CA125 were both slightly decreased in 40-49 age group and both reference ranges are slightly higher than previously reported [14,15,25,27]. However, in laboratory statistics, a minimum of 120 healthy subjects are generally required to establish reference range, and we analyzed 200 healthy individuals.
In this study, we established reference ranges for HE4 and CA125 according to age, which can be useful for interpreting the results. The performance study was also evaluated to validate the utility of the automated HE4 assay. However, we could not obtain information on menopausal status and we could not include a patient group with malignant or non-malignant gynecologic diseases. Therefore, we could only establish reference ranges according to age in healthy individuals. Nevertheless, our study showed that HE4 and CA125 both had age-related variability, CA125 levels were significantly lower in older age groups (60 years and above), and there was no correlation between HE4 and CA125.
Therefore, we suggest that using both biomarkers (HE4 and CA125) for screening and diagnosis with reference range according to age, if the CA125 result was ambiguous in a routine laboratory test for gynecological diseases. Further studies on reference range according to menopausal status in various ethnic groups and diagnostic performance in comparison with CA125 for various patient groups with malignant and non-malignant gynecologic diseases are necessary for clinical use of the HE4 assay.
In conclusion, the automated HE4 assay showed
good analytical performance, age-related variable results and no correlation with CA125. HE4 in combination with CA125 is likely to be a more useful diagnostic tool than reliance on CA125 alone.
요 약
배경: 난소암의 검사실적 진단은 CA125의 정량적 측정 에 기초하고 있다. 수년간 난소암에 대한 다양한 표지자들이 많이 제안되어 연구되어 왔고 그 중에서 human epididymis protein 4 (HE4)가 새로운 난소암의 표지자로 나타났다. 새로운 HE4 자동화 분석장비를 평가하기 위해 분 석적 수행능을 평가하고 참고치를 측정하였다.
방법: 새로운 HE4 자동화 분석장비의 분석적 수행능에 대해 평가를 실시하였고 HE4와 CA125의 나이에 따른 참고 치를 측정하였다. HE4와 CA125의 상관성에 대해서도 분석 하였다.
결과: 저농도와 고농도 물질을 사용한 정밀도 실험에서는 높은 정밀도를 보여주었다. 건강인의 95% 정상 참고 범위의 상한값은 HE4와 CA125에서 각, 81.0 pmol/L (90% CI, 63.0-103.1)과 28.6 U/mL (90% CI, 25.4-36.4)였다.
건강한 여성에서 HE4와 CA125 사이에는 상관관계가 없었 다(r = -0.002,P = 0.9793). HE4의 정상 참고 범위의 상한값은 나이가 많은 군에서 나이가 어린 군에 비해 약간 증 가하는 경향을 보였다. CA125는 더 다양한 값을 보였고 특 히 나이가 많은 그룹에서는 크게 감소하였다.
결론: 새로운 HE4 자동화 분석장비의 분석적 수행능은 우수하였으며 나이에 따라 결과값이 다르고 CA125와의 관 련성은 없었다. 실제 임상에서 난소암의 진단 및 선별검사에 서의 임상적 유용성에 대한 추가적인 연구가 더 필요하지만, HE4와 CA125를 함께 측정하는 것이 CA125를 단독으로 측정하는 것보다 더 유용할 것으로 생각된다.
REFERENCES
1. Jemal A, Siegel R, W ard E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49.
2. Aebi S, Castiglione M; ESMO Guidelines Working Group.
Newly and relapsed epithelial ovarian carcinoma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20(S4):S21-3.
3. Ministry for Health, Welfare and Family Affairs. Annual report of cancer incidence (2007), cancer prevalence (2007) and survival (1993-2007) in Korea. Seoul: Ministry for Health, W elfare and Family Affairs; 2009.
4. Brewster WR. Temporal trends in ovarian cancer: incidence and mortality across Europe. Nat Clin Pract Oncol 2005;2:286-7.
5. Kim K, Zang R, Choi SC, Ryu SY, Kim JW . Current status
of gynecological cancer in China. J Gynecol Oncol 2009;20:72-6.
6. Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006;98:172-80.
7. Paulsen T, Kjaerheim K, Kaern J, Tretli S, Trope C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer 2006;16(S1):S11-7.
8. Carney ME, Lancaster JM, Ford C, Tsodikov A, W iggins CL.
A population-based study of patterns of care for ovarian cancer:
who is seen by a gynecologic oncologist and who is not? Gynecol Oncol 2002;84:36-42.
9. Maggino T, Gadducci A, D'Addario V, Pecorelli S, Lissoni A, Stella M, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol 1994;54:117-23.
10. Kobayashi H, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int J Gynecol Cancer 2008;18:414-20.
11. Bast RC Jr. Status of tumor markers in ovarian cancer screening.
J Clin Oncol 2003;21(10S):200s-205s.
12. Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 2003;63:3695-700.
13. Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass.
Gynecol Oncol 2008;108:402-8.
14. Park Y, Kim Y, Lee EY, Lee JH, Kim HS. Reference ranges for HE4 and CA125 in a large Asian population by automated assays and diagnostic performances for ovarian cancer. Int J Cancer. 2012;130:1136-44.
15. Li J, Dowdy S, Tipton T, Podratz K, Lu WG, Xie X, et al. HE4 as a biomarker for ovarian and endometrial cancer management.
Expert Rev Mol Diagn 2009;9:555-66.
16. Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods;
Approved Guideline-Second Edition. CLSI document EP5-A2.
Wayne: Clinical and Laboratory Standards Institute, 2004.
17. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; Approved Guideline. CLSI document EP6A. Wayne:
Clinical and Laboratory Standards Institute, 2003.
18. Schink JC. Current initial therapy of stage III and IV ovarian cancer: challenges for managed care. Semin Oncol 1999;26(S1) :S2-7.
19. Rosen DG, W ang L, Atkinson JN, Yu Y, Lu KH, Diamandis
EP, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol 2005;99:267-77.
20. Gadducci A, Ferdeghini M, Prontera C, Moretti L, Mariani G, Bianchi R, et al. The concomitant determination of different tumor markers in patients with epithelial ovarian cancer and benign ovarian masses: relevance for differential diagnosis.
Gynecol Oncol 1992;44:147-54.
21. Kim JH, Skates SJ, Uede T, W ong KK, Schorge JO, Feltmate CM, et al. Osteopontin as a potential diagnostic biomarker for ovarian cancer. JAMA 2002;287:1671-9.
22. Healy DL, Burger HG, Mamers P, Jobling T, Bangah M, Quinn M, et al. Elevated serum inhibin concentrations in postmenopausal women with ovarian tumors. N Engl J Med 1993;329:1539-42.
23. Lenhard MS, Nehring S, Nagel D, Mayr D, Kirschenhofer A, Hertlein L, et al. Predictive value of CA 125 and CA 72-4 in ovarian borderline tumors. Clin Chem Lab Med 2009;47:537-42.
24. Montagnana M, Lippi G, Ruzzenente O, Bresciani V, Danese E, Scevarolli S, et al. The utility of serum human epididymis protein 4 (HE4) in patients with a pelvic mass. J Clin Lab Anal 2009;23:331-5.
25. Halila H, Stenman UH, Seppala M. Ovarian cancer antigen CA 125 levels in pelvic inflammatory disease and pregnancy. Cancer 1986;57:1327-9.
26. Malkasian GD Jr, Knapp RC, Lavin PT, Zurawski VR Jr, Podratz KC, Stanhope CR, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease.
Am J Obstet Gynecol 1988;159:341-6.
27. Park Y, Lee JH, Hong DJ, Lee EY, Kim HS. Diagnostic performances of HE4 and CA125 for the detection of ovarian cancer from patients with various gynecologic and non-gynecologic diseases. Clin Biochem 2011;44:884-8.
28. Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, et al. Use of a Symptom Index, CA125, and HE4 to predict ovarian cancer. Gynecol Oncol 2010;116:378-83.
29. Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009;112:40-6.
30. Bon GG, Kenemans P, Verstraeten R, van Kamp GJ, Hilgers J. Serum tumor marker immunoassays in gynecologic oncology:
establishment of reference values. Am J Obstet Gynecol 1996;174:107-14.
31. Ruggeri G, Bandiera E, Zanotti L, Belloli S, Ravaggi A, Romani C, et al. HE4 and epithelial ovarian cancer: comparison and clinical evaluation of two immunoassays and a combination algorithm. Clin Chim Acta 2011;412:1447-53.