관련 문서
this study analysed cast speed and solidification in thermal and flow perspectives and based on the results, conducted a confidence test on the high-speed general purpose
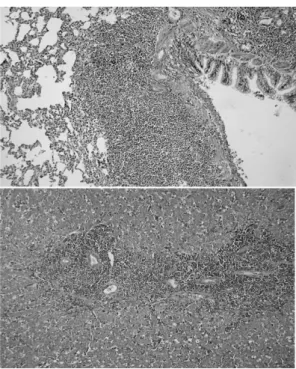
We investigated upper gastrointestinal tract involvement and characteristic endoscopic findings in scrub typhus, and we also determined the correlation between
This paper is a case study on how Venture and Small-Business Executives managers can take advantage of their intuitions in situations where the
This chapter aims to discuss the organic relations between Shakespeare’s literature and Star Trek series based on The Sonnets that is characteristic of the
Second, the seventh educational course applied to the practical curriculum in 2001, and it applied to the first- grade students of high school , so as
Just as we achieved good control with a joint-based controller that was based on a linearizing and decoupling model of the arm, we can do the same for the Cartesian case.
출처 : IAEA 발표 자료(Comprehensive inspection exercise at bulk handling facilities, “U-235 Enrichment measurements by gamma-ray spectroscopy”) 13. Uranium
Based on the findings of this study, it was considered that the senior welfare centers should manage and operate various health promotion programs to