Osteosarcoma (OS) is the most common primary malig- nant tumor of the bone and this disease primarily afflicts individuals in the second and third decade of life. Many helpful chemotherapeutic regimens are currently being used for the treatment of OS, and they have improved the survival rates in recent years8). Yet there still patients who don’t res- pond to chemotherapy13). Moreover, the toxic and adverse effects associated with chemotherapy can significantly reduce the quality of a patient’s life4). Therefore, more efficacious therapeutic drugs are needed to improve the quality of a patient’s life and the long-term OS survival rate.
Recent studies have proposed using Peroxisome Prolifer- ator-Activated Receptor (PPAR) ligands as a new chemo- therapeutic agents to treat human malignant tumors. PPAR is expressed mainly in adipose tissue, and it is also found in osteoblasts, cartilage, myocytes, hepatocytes, fibroblasts, endothelial cells, epithelial cells, and many types of cancer
cells2,3,5). Recent studies have showed that PPAR ligands, such as troglitazone, pioglitazone and ciglitazone exhibit growth inhibitory effects by inducing the differentiation and apoptosis, or by inhibiting angiogenesis in a variety of human malignant cells12).
This study investigated the relationship between the effects of TRO on the cellular growth in two human OS cell lines (U2OS and HOS).
MATERIALS AND METHODS
1. Ligands and cell culture
Troglitazone (TRO) was obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA). The stock solution was prepared at a concentration of 100 mmol/L in ethanol.
The human osteosarcoma cell lines HOS and U2OS were obtained from the Korean Cell Line Bank (KCLB) and they were cultured in RPMI 1640 (HOS) or McCoy’s 5A medi- um (U2OS) that contained 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 mg/mL) in a water-jacketed incubator with a humidified atmosphere (5% CO2, 95% air) at 37℃. In all the experi- ments, the cells were left to recover from trypsinization and they were treated 24 hours after being plated. The amount
591 591
Effects of the Peroxisome Proliferator-Activated Receptor Ligand Troglitazone in Osteosarcoma Cell Lines
Yeung Jin Kim, M.D., Tae Kyun Kim, M.D., Jin Young Park, M.D., Hyoung Joon Kim, M.D., and Ji Wan Lee, M.D.
Department of Orthopedic Surgery, College of Medicine, Wonkwang University Hospital, Iksan, Korea
591 591 Address reprint requests to
Tae Kyun Kim, M.D.
Department of Orthopedic Surgery, College of Medicine, Wonkwang University 344-2 Shinyong-dong, Iksan 570-711, Korea
Tel: +82.63-472-5100, Fax: +82.63-472-5104 E-mail: yjkim1@wonkwang.ac.kr
*The author has received support from the Wonkwang University Research Foundation.
Purpose: We wanted to investigate the effects of Troglitazone (TRO) on the cellular growth in two human osteosarcoma cell lines (U2OS and HOS).
Materials and Methods: Cell viability was assessed by performing trypan blue exclusion assay. FAC- Scan analysis was performed to study the cell cycle and apoptosis. Antibodies against PTEN, tAkt, pAKt, Bcl-2, Bax, pRB, p21Cip1, and -actin were used in the Western blot analysis.
Results: TRO inhibited the growth of both the osteosarcoma cell lines. TRO induced G0/G1 arrest in the cell cycle progression for both osteosarcoma cell lines. TRO induced the apoptosis of HOS cells, but it decreased apoptosis of the U2OS cells. TRO induced Rb dephosphorylation and the increased expression of p21Cip1. TRO increased the PTEN and Bcl-2 expressions and it decreased the level of pAkt, pRb and Bax.
Conclusion: The present study suggested that TRO may be used as a chemotherapeutic agent for the treatment of human OS. Yet further study is required for uncovering the precise the mechanism of TRO.
Key Words: Osteosarcoma, Troglitazone (TRO)
of ethanol added as the vehicle never exceeded 0.1% of the total volume.
2. Effects of troglitazone on the growth of osteosarcoma cell lines
Each cell line was seeded at 1×105cells/mL per 100 mm on a culture plate. The culture medium was replaced with fresh medium and then TRO or ethanol was added after 24 hours. After 48 hours, the adherent cells were detached with trypsin/EDTA, and the cell number was determined with using a hemocytometer. Cell viability was assessed by trypan blue exclusion assay.
3. FACScan analysis of cell cycle and apoptosis For the cell cycle analysis, the cells were plated at an initial density of 1×106cells/mL per 100 mm onto culture plates.
The culture medium was replaced with fresh medium and then TRO or ethanol was added after 24 hours. After wash- ing with PBS, the cells were incubated with propidium iodide (Sigma Chemical Corporation, St. Louis, MO, USA) stain- ing solution at room temperature for 1 hour in the dark.
The stained cells were analyzed by using a FACStar flow cytometer (Becton-Dickinson, San Jose, CA). The percent- ages of the cells in the sub-G1, G0/G1, S and G2/M phas- es were determined by cell Quest software (Becton-Dick- inson).
4. TUNEL assay
To study the apoptosis of the cultured cells, TUNEL (ter- minal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling of DNA fragmentation sites) assay was
performed. 5×105cells/slide were incubated in the presence or absence of 20 M TRO as an immunohistochemical probe on the slides for 48 hours. Then counted the total cells and the TUNEL-positive cell in ten randomly chosen high power fields. The cells containing condensed chromatin were count- ed as cells experiencing apoptosis. Positive and negative con- trols were performed using the cells treated with 1 lg/mL DNase I and using the cells without terminal deoxynucleo- tidyl transferase treatment.
5. Western blot analysis
The concentrations of the total proteins in the extracts were determined by using the Bradford protein assay, and bovine serum albumin (BSA) was used as a protein standard.
Cell extracts (30 g of the protein/lane) were separated on 6- 12% SDS-polyacrylamide gels with the Tri-glycine buffer system, and the proteins were transferred to polyvinyliden difluoride (PVDF) membranes (Boehringer Mannheim, Germany) with using a semi-dry transfer system as recom- mended by the manufacturer (Bio-Rad, Hercules, CA). The following primary antibodies were used in this study: anti- bodies against PTEN (Upstate, IL, USA), tAkt, pAKt (Cell Signaling, MA, USA), Bcl-2, Bax, pRB, p21Cip1(Santa Cruz, CA, USA), and -actin (Sigma, USA).
6. Statistical analysis
The t test was used to determine the statistical significance of the obtained data and to compare the means of the two groups. One-way ANOVA and the Student-Newman-Keuls test were used for multiple comparisons. p values<0.05 were considered statistically significant.
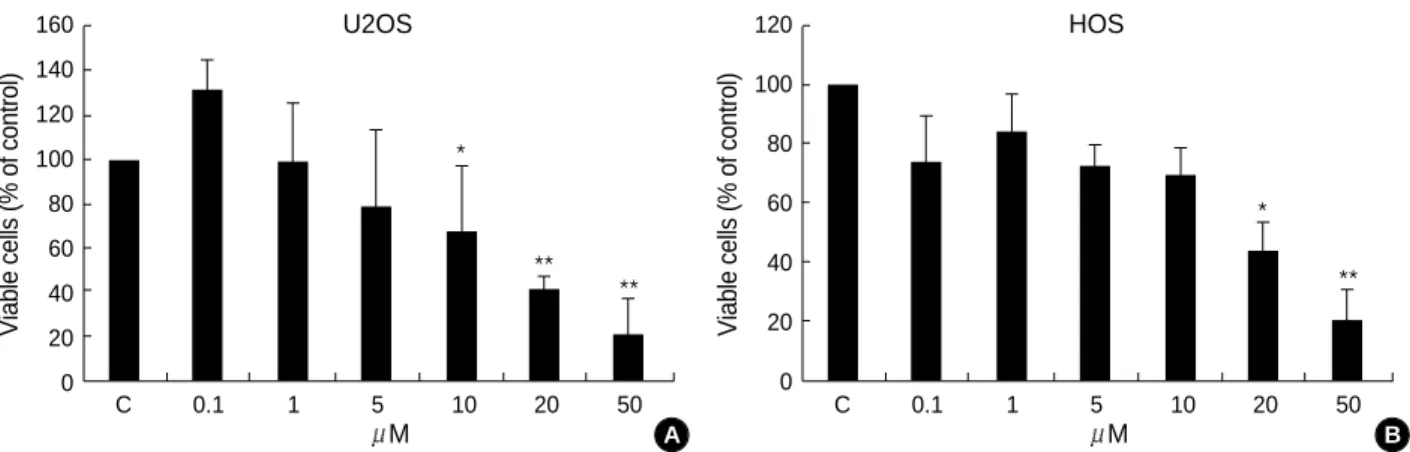
Fig. 1.The TRO-mediated growth effects in human osteosarcoma cells. U2OS and HOS osteosarcoma cells were treated with either ethanol alone or with different concentrations of TRO, as indicated.
Viable cells (% of control)
160 140 120 100 80 60 40 20
0 C 0.1 1 5 10 20 50
U2OS
M
Viable cells (% of control)
120 100 80 60 40 20 0
C 0.1 1 5 10 20 50
HOS
M
*
**
*
** **
A B
RESULTS
1. Troglitazone inhibits the growth of osteosarcoma cell lines
As shown in Fig. 1, inhibition of cell growth was observed in the U2OS and HOS cells treated with TRO. Inhibition of cell growth was evident at the concentration of 10 M of TRO in the U2OS cells and at the concentration of 20
M of TRO in HOS cells.
2. Troglitazone induces G0/G1 arrest in the cell cycle progression for the osteosarcoma cell lines
When the OS cells were treated with TRO at the concen- tration of 5 M, 10 M, 20 M, and 50 M during the 48 hours, the proportion of cells in the G1 phase was increased (Fig. 2, 3). These result indicate that the growth inhibition of the OS cells following TRO treatment can be attributed to arrest in the G1 phase.
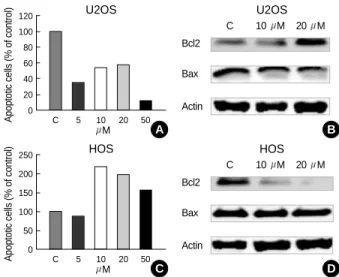
3. Troglitazone induced apoptosis of the HOS cells, but it decreased apoptosis of the U2OS cells
As shown in Fig. 4, the TRO effects on apoptosis showed different results according to the cell lines. The apoptosis of the U2OS cells that naturally occurred in the culture was decreased by treatment of TRO. On the other hand, the apop- tosis of the HOS cells were increased by TRO treatment at the concentrations of 10 M, 20 M, and 50 M. To fur- ther examine this, we performed TUNEL analysis to eval- uate the apoptotic effects of TRO. The results of the TUNEL analysis were identical with the flow cytometric analysis (Fig. 5). These two independent methods of measuring apop- tosis provided evidence that the effects of TRO on the apop- tosis were different depending on the cell lines. As shown in Fig. 4, U2OS cells treated with 10 M and 20 M of TRO during 48 hours showed a gradually increased expres- sion of Bcl-2 and a mildly decreased expression of Bax. On the other hand, the expression of Bcl-2 was significantly decreased in the HOS cells treated with 10 M and 20 M of TRO in a dose-dependent manner. These results indicate that the decreased apoptosis of the TRO treated U2OS cells was mediated by the increased expression of Bcl-2 and the decreased expression of Bax. On the contrary, TRO acts as an apoptosis inducing factor to the HOS cells by decreasing
Fig. 2.TRO induced G1 cell cycle arrest and it decrease of apop- tosis in the U2OS cells.
300
0
0 200 400 600 800 1,000 Control Sub-G1 21.52%
G0/G1 38.69%
S 25.82%
G2-M 13.97%
300
0
0 200 400 600 800 1,000 TRO 5 M Sub-G1 7.56%
G0/G1 49.36%
S 28.29%
G2-M 14.79%
300
0
0 200 400 600 800 1,000 Sub-G1 11.64%
G0/G1 45.68%
S 26.33%
G2-M 16.35%
300
0
0 200 400 600 800 1,000 TRO 20 M TRO 10 M
300
0
0 200 400 600800 1,000 Sub-G1 2.56%
G0/G1 48.04%
S 32.06%
G2-M 17.34%
TRO 50 M Sub-G1 12.65%
G0/G1 47.96%
S 23.84%
G2-M 15.55%
U2OS
Fig. 3.TRO induced G1 cell cycle arrest and increase of apop- tosis in the HOS cells.
300
0
0 200 400 600 800 1,000 Control Sub-G1 5.19%
G0/G1 27.59%
S 61.34%
G2-M 5.88%
300
0
0 200 400 600 800 1,000 TRO 5 M Sub-G1 4.56%
G0/G1 30.83%
S 58.31%
G2-M 6.40%
300
0
0 200 400 600800 1,000 Sub-G1 11.55%
G0/G1 29.45%
S 50.15%
G2-M 8.85%
300
0
0 200 400 600 800 1,000 TRO 20 M TRO 10 M
300
0
0 200 400 600 800 1,000 Sub-G1 8.24%
G0/G1 37.16%
S 32.85%
G2-M 21.75%
TRO 50 M Sub-G1 10.42%
G0/G1 28.49%
S 54.11%
G2-M 6.99%
HOS
Fig. 4.The TRO effects of apoptosis of TRO showed different results according to the cell lines. (A) Apoptosis of the U2OS cells was decreased by TRO treatment. (B) U2OS cells showed a gradually increased expression of Bcl-2 and a mildly decreased expression of Bax by western blot analysis. (C) On the other hand, apoptosis of the HOS cells were increased by TRO treat- ment. (D) The expression of Bcl-2 was significantly decreased in the HOS cells.
Apoptotic cells (% of control)
120 100 80 60 40 20 0
C 5 10 20 50
Bcl2
C 10 M 20 M
Bax
Actin
U2OS U2OS
M
Apoptotic cells (% of control)
250 200 150 100 50 0
C 5 10 20 50
Bcl2
C 10 M 20 M
Bax
Actin
HOS HOS
M
A B
C D
the expression of Bcl-2. 4. Troglitazone induces Rb dephosphorylation and it increases the expression of p21Cip1
Hyperphosphorylation of Rb is a critical event in the G1 phase which allows cells to pass through a cell cycle check point and then enter the S phase. Rb exists in an active hy- pophosphorylated state in quiescent cells and in an inactive hyperphosphorylated state in the G1/S cell-cycle transition.
As shown in Fig. 6, TRO treatment decrease the expression of phosphorylated Rb in both the U2OS and HOS cells. The expression of another cell cycle regulator, p21Cip1was increased in both the U2OS and HOS cells after treatment with 10 M and 20 M of TRO for 48 hours. When combined, these data suggest that the induction of G1 arrest in OS cell lines by TRO was caused by hypophosphorylation of Rb and the increased expression of p21Cip1.
5. Troglitazone increases PTEN expression
PTEN activity causes cell cycle arrest and apoptosis. It has been proposed that PTEN blocks the cell cycle by increas- ing the transcription of the p27 cell cycle inhibitor. With the loss of PTEN, therefore, cells are released into the cell cycle. As shown in Fig. 7, TRO increased the PTEN expres- sion and decreased the level of phosphorylated Akt.
DISCUSSION
Recent studies have shown PPAR activation act as a tu- mor suppressive agent by inducing cell cycle arrest, apoptosis or differentiation in several human malignant tumor2,3,5,12). However, conflicting evidence exists on the role of PPAR
Fig. 6.TRO treatment induces the increased expression of p21 and decreased expression of phosphorylated Rb (pRb) protein.
A p21
pRB actin
C 10 M 20 M
U2OS
B p21
pRB actin
C 10 M 20 M
HOS
Fig. 7.TRO treatment induces the increased expression of PTEN and the decreased expression of phosphorylated Akt (pAkt) pro- tein in both the U2OS and HOS cells.
A PTEN
tAkt pAkt
C 10 M 20 M
U2OS
B PTEN
tAkt pAkt
C 10 M 20 M
HOS Fig. 5.Effects of TRO on the induction of apoptosis in U2OS
and HOS cells. (A) The cells were stained for apoptotic cells and these were photographed under a light microscopy. The cells containing light brown nuclei were positive for TUNEL assay.
(B) The apoptosis of U2OS cells was decreased by a TRO treat- ment. On the other hand, the apoptosis of HOS cells was incre- ased by TRO treatment.
A
B Control
U2OS HOS
TRO 20 M
TUNEL-positive cells/ 1,000 tumor cells 7 6 5 4 3 2 1
0 C 20
U2OS
M
TUNEL-positive cells/ 1,000 tumor cells 12 10 8 6 4 2 0
C 20
HOS
M
*
*
Treatment TUNEL-positive cells/1,000 tumor cells
U2OS HOS
Control 5.08±0.78 3.44±0.36
TRO 20 M 0.68±0.27* 9.88±1.01*
activation in OS, where different studies have shown that PPAR activation affected the OS survival or, in contrast, it induced apoptosis and differentiation of the OS3).
In this study, TRO induced growth inhibition and G1 phase arrest in both the U2OS and HOS cells by upregu- lating the expression of p21 and decreasing the phosphory- lated Rb. The physiologic role of p21 has been linked to the inhibition of cyclin E/CDK27,11).
In addition to the upregulation of p21 and the downreg- ulation of phosphorylated Rb, TRO treatment also increased the expression of PTEN. The loss of PTEN activity has been suggested to cause enhanced cell proliferation14). A previous study showed that inhibition of PTEN activity resulted in increased levels of Akt phosphorylation and enhanced osteo- sarcoma cell proliferation10). Another study also demonstrated that PPAR ligands increase the PTEN expression in pan- creatic cancer cells2). The present study also supports the pre- vious studies that TRO induced the upregulation of PTEN and this has a role in the growth inhibition of OS cells.
Many previous reports have also shown that TRO induced the growth inhibition and G1 cell cycle arrest of human can- cer. However, there are different mechanism of cell cycle arrest, depending on the target cell type, the particular PPAR agonist used, the duration of treatment, and the applied dosage1,3,5).
The improper balance between death and growth in tumor cells can involve several signals, so we can ask this question:
what signaling pathways are critical for OS growth and death?
This study showed that although apoptosis was decreased in the U2OS cells by treatment with TRO, the overall tumor cell growth was inhibited in a dose dependent fashion. The growth inhibitory effect of TRO was not evident at treat- ment concenturation of 0.1, 1, and 5 M TRO, but 10, 20, and 50 M of TRO significantly inhibited cell growth in a dose dependent fashion. It has been suggested that the other signals inducing cell cycle arrest such as pRb, p21, and the PTEN/Akt pathway may act more potently for inducing growth inhibition of U2OS cells. In the study using mela- noma cell lines, the growth inhibitory effect of the PPA- PPAR ligands were independent of apoptosis and they seemed to occur primarily through the induction of cell cycle arrest9).
In the present study, TRO treatment on the U2OS cell increased the expression of p21 and PTEN. Among these,
p21 is controlled by p53 and the increased expression of p53 induces upregulation of p21; this resulted in increased apoptosis. Yet the increased expression of p21 was not in accordance with the reduced apoptosis of U2OS cells. This result suggested that p53 the mediated effects on the p21 pathway were not important in the apoptosis of U2OS cells.
A recent study supported this assumption. Kim et al.6)have suggested that p53 overexpression is required but it is not sufficient enough for inducing apoptosis of the U2OS cells.
Therefore, there are other mechanisms other than p53, and TRO act in a different manner according to cell type. Fur- ther research is necessary to define the molecular mechanisms of the on the TRO growth inhibitory effects, and especially for the mechanism of apoptosis.
TRO inhibits osteoclast-like cell formation and bone re- sorption. The inhibitory effects of TRO on osteoclastic bone resorption were not osteotropic factor specific and they did not appear to be related to their adipogenic effects. Thus, TRO may suppress bone resorption and prevent bone loss.
PPAR activators modulate the osteoblastic maturation of MC3T3-E1 preosteoblasts. PPAR activators may affect BMD by modulating the maturation of osteoblasts15).
Unfortunately, TRO has side effects, including edema and severe hepatotoxicity when it is administered at the high doses. The processing of the quinone metabolite of TRO which is found predominantly in the liver to a sulfate con- jugate and the activation of PPAR gamma and PXR (preg- nane X receptor) by TRO are supposed to be factors of the hepatotoxic mechanism. It has been recommended to exam- ine every month the liver function in patients treated with the this drug in order detect drug-induced hepatitis at an early stage16).
CONCLUSION
The present study suggest that TRO may be used as an efficacious adjuvant chemotherapeutic agent for primary osteosarcoma, as well as that TRO may be used as a poten- tial chemopreventive agent for preventing the recurrence and metastasis of osteosarcom after the surgical removal of primary tumors. But further study is required for elucidat- ing the precise the mechanism of TRO.
REFERENCES
1. Bae MA, Rhee H and Song BJ: Troglitazone but not rosiglita-
zone induces G1 cell cycle arrest and apoptosis in human and rat hepatoma cell lines. Toxicol Lett, 139: 67-75, 2003.
2. Farrow B and Evers BM: Activation of PPAR increases PTEN expression in pancreatic cancer cells. Biochem Biophys Res Com- mun, 301: 50-53, 2003.
3. Haydon RC, Zhou L, Feng T, et al: Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma.
Clin Cancer Res, 8: 1288-1294, 2002.
4. Ibrahim S, Sundari MN and Masir N: Osteosarcoma in a six- teen-month old boy. Med J Malaysia, 54: 261-263, 1999.
5. Keshamouni VG, Reddy RC, Arenberg DA, et al: Peroxisome proliferators-activated receptor- activation inhibits tumor progres- sion in non-small-cell lung cancer. Oncogene, 23: 100-108, 2004.
6. Kim M, Sgagias M, Deng X, et al: Apoptosis induced by aden- ovirus-mediated p14ARFexpression in U2OS osteosarcoma cells is associated with increase Fas expression. Biochem Biophys Res Com- mun, 320: 138-144, 2004.
7. Koff A, Giordano A, Desai D, et al: Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science, 257: 1689-1694, 1992.
8. Meyers PA, Heller G and Healey J: Chemotherapy for non- metastatic osteogenic sarcoma: The Memorial Sloan-Kettering expe- rience. J Clin Oncol, 10: 515, 1992.
9. Mossner R, Schulz U, Kruger U, et al: Agonists of peroxisome
proliferators-activated receptor inhibit cell growth in malignant melanoma. J Invest Dermatol, 119: 576-582, 2002.
10. Nielsen-Preiss SM, Silvia SR and Gillette JM: Role of PTEN and Akt in the regulation of growth and apoptosis in human osteo- blastic cells. J Cell Biochem, 90: 964-975, 2003.
11. Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM and Pagano M: Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol, 15: 2612-2624, 1995.
12. Panigrahy D, Singer S, Shen LQ and ET AL: PPAR ligands inhibit primary tumor growth and metastasis by inhibiting angio- genesis. J Clin Invest, 110: 923-932, 2002.
13. Scotlandi K, Serra M and Nicoletti G: Multidrug resistance and malignancy in human osteosarcoma. Cancer Res, 56: 2434-2439, 1996.
14. Stambolic V, Suzuki A, de la Pompa JL, et al: Negative reg- ulation of PKB/Akt-dependent cell survival by the tumor suppres- sor PTEN. Cell, 95: 29-39, 1998.
15. Tornvig L, Mosekilde LI, Justesen J, Falk E and Kassem M: Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif Tissue Int, 69: 46-50. 2001.
16. Toyota T and Ueno Y: Clinical effect and side effect of troglita- zone. Nippon Rinsho, 58: 376-382, 2000.
. . . .
목 적: U2OS와 HOS 골육종 세포주에 대한 Troglitazone (TRO)의 영향을 알아보고자 하였다.
대상 및 방법: TRO를 처리한 골육종 세포의 생활력은 trypan blue exclusion을 이용하여 측정하였고, 세포주기 분포는 흐 름세포측정을 이용하여 판정하였다. 세포자멸사는 흐름세포측정과 TUNEL 분석법을 이용하여 측정하였다. 세포자멸사와 세포 주기에 관련된 유전자의 발현은 PTEN, tAkt, pAKt, Bcl-2, Bax, pRB, p21Cip1, and -actin을 이용한 웨스턴 블롯으로 평가하였다.
결 과: U2OS와 HOS 세포주 모두에서 세포 성장의 억제를 보였고, 흐름세포측정에서도 모두에서 G1기에서 세포주기 정지 가 관찰되었다. 또한 p21Cip1과 PTEN 발현은 증가하였고 pRb와 pAkt의 발현은 감소하였다. 그러나 흐름세포측정과 TUNEL 분석법을 이용하여 세포자멸사를 측정한 결과 서로 다른 결과를 보였다. HOS 세포주의 세포자멸사는 증가한 반면 U2OS 세포주의 세포자멸사는 감소하였다. U2OS 세포주에서 세포자멸사의 감소는 Bcl-2 발현의 증가 및 Bax 발현의 감소와 함 께 관찰되었다.
결 론: TRO가 작용하는 기전이 세포주에 따라 차이가 있을 수 있으나 적절하게 입증된 용량으로 사용한다면 골육종 치료 에 이용될 수 있는 가능성을 보여주는 것으로 생각된다.
색인 단어: 골육종, Troglitazone (TRO)
인간 골육종세포에 대한 Peroxisome Proliferator-Activated Receptor 배위자 Troglitazone의 영향
김영진ㆍ김태균ㆍ김형준ㆍ박진영ㆍ이지완
원광대학교 의과대학 정형외과학교실