Received: 3 September, 2012 Revised: 24 October, 2012 Accepted: 31 October, 2012 Corresponding author: Jerrold Petrofsky
Department of Physical Therapy, Loma Linda University, Loma Linda, California 92350, USA Tel: 1-909-558-7274 Fax: 1-909-558-0481 E-mail: jpetrofsky@llu.edu
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licens es/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Copyright © 2012 Korean Academy of Physical Therapy Rehabilitation Science
pISSN 2287-7576 Phys Ther Rehabil Sci
eISSN 2287-7584 2012, 1 (1), 6-12
www.jptrs.org
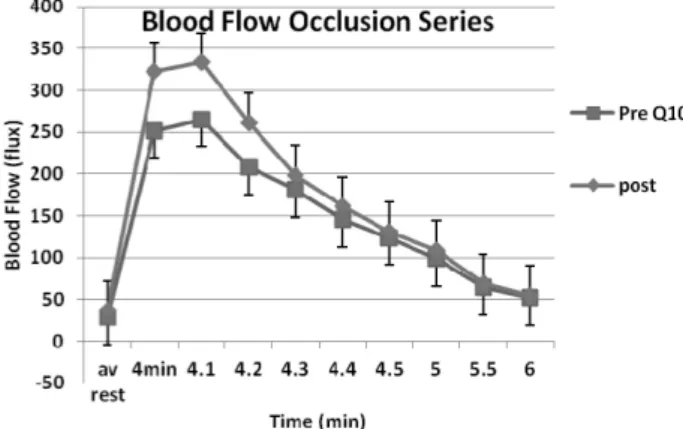
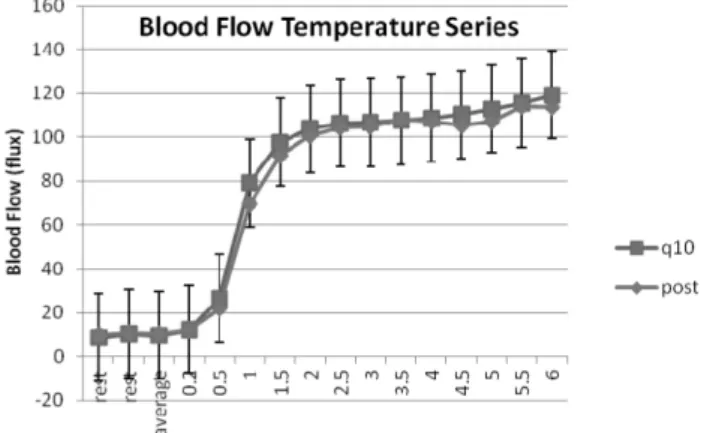
The effect of coenzyme Q10 on endothelial function in a young population
Jerrold Petrofsky a,b , M. Laymon a , H. Lee b , E. Hernandez a , D. Dequine a , L. Thorsen a , R. Lovell a , J. Andrade a
a
Department of Physical Therapy, Azusa Pacific University, CA, USA
b