Received: June 17, 2016, 2016 / Revised: August 8, 2016 / Accepted: August 14, 2016 Address for correspondence: Joong-Yang Cho, MD, PhD
Department of Neurology, Ilsan Paik Hospital, Inje University College of Medicine, 170, Juhwa-ro, Ilsanseo-gu, Goyang 10380, Korea Tel: +82-31-910-7929, Fax: +82-31-910-7368, E-mail: joongyangcho@gmail.com
Correlation of Electrophysiologic Findings with Clinical Severity Scales in Carpal Tunnel Syndrome
Ji Eun Kim, MD1, Joong-Yang Cho, MD, PhD2
1Department of Neurology, Asan Medical Center, Ulsan University College of Medicine, Seoul; 2Department of Neurology, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
KEYWORDS
Carpal tunnel syndrome, Electrophysiologic severity, Symptom assessment
Background: This study aims to investigate the relationship between electrophysiologic severity and several clinical severity scales in patients with carpal tunnel syndrome (CTS).
Methods: Two hundred patients with documented CTS were enrolled. The severity of electro- physiologic impairment was classified by Padua scale. The main outcome measures were as- sessed with several clinical scales which were categorized into two groups: Patient questionnaire consisted of Symptom Severity Scale (SSS), Functional Status Scale (FSS), and faces pain scale.
Physician-based quantitative clinical scale was Historical-Objective (Hi-Ob) scale. The electro- physiologic severity was compared with clinical scales, respectively.
Results: Clinical severity assessments using SSS, FSS and pain scale showed weak correlation with electrophysiologic severity of CTS. However, Hi-Ob scale was moderately correlated with electrophysiologic severity of CTS.
Conclusions: Electrophysiologic severity of CTS was more correlated with physician-measured clinical scale than patient-oriented symptom scales.
Introduction
Carpal tunnel syndrome (CTS) is the most common com- pressive neuropathy in clinical practice, characterized by entrapment of the median nerve at wrist. Cardinal symptoms of CTS such as paresthesia, pain with nocturnal exacerbation and numbness are common in the distribution of the median nerve. Its incidence is reported up to approximately 3-6 per- cent of adults in general population and more common in middle-aged women.1,2 The risk of CTS is higher in occupa- tions exposed to high pressure, high force in the wrist by re- petitive tasks and posture.3
A universally agreed-on reference standard test for the di- agnosis of the CTS is absent. However, clinicians use 1 of following 3 methods generally: (1) symptoms and signs
alone, (2) electrophysiologic studies, or (3) both.4
The optimal method is debatable, but electrophysiologic stud- ies have well-reported diagnostic performance characteristics.5 Differential diagnosis that need other management such as brachial plexopathy and peripheral polyneuropathy can be excluded by electrophysiologic studies. In this regard, utility of electrophysiologic study as an essential tool for excluding other conditions mimicking CTS has been emphasized.6 Electrophysiologic study is also helpful in deciding ther- apeutic plan based on disease severity. However, there are conflicting results about the correlation between severity of electrophysiologic findings and symptoms in patients with CTS.6-10 Thus we evaluated the correlation between electro- physiologic severity and several clinical severity scales in patients with CTS to find the most reliable and correlative one.
Materials and Methods
1. Data collection and electrophysiologic grading
This cross sectional single center study was conducted for consecutive patients who visited our clinic with suspected sensory and/or motor symptoms of CTS from March 2011 to November 2013. This study was approved by the local med- ical ethics committee. All patients with suspected CTS were interviewed for detailed history and examined to check sen- sory change of hand, weakness of thumb on palmar abduc- tion, or thenar atrophy. Tinel’s and Phalen’s testings were al- so performed.
Electrophysiologic studies were carried out according to the protocol inspired by the American Association of Elec- trodiagnostic Medicine Recommendations (using Nicolet Viking select EMG/NCS Machine with bipolar stimulating electrodes, Nicolet Medical, San Carlos, CA, USA).11 Studies were always done in the same place and in the similar room temperature (patients’ skin temperature: 32-34°C).
Study protocol included several commonly used nerve conduction parameters as follows that have high degree of sensitivity and specificity: (1) abnormal sensory nerve con- duction in the finger-wrist segment (reference value ≥41.26 m/s); (2) abnormal sensory nerve conduction in palm-wrist segment (reference value ≥34.05 m/s); and (3) prolonged terminal latency (reference value ≤3.60 msec).12
According to the protocol, motor and sensory nerve con- duction study (NCS) of median and ulnar nerve was per- formed in both hands. Additionally, bilateral evaluation was done for comparison, in case of patients with unilateral symptom. The NCS results of each patient were analyzed and graded according to the Padua severity scale.13 In pa- tients who complain of symptoms in both hands, we used re- sult of the more severe hand, rather than averaging of the two hands measurements, for statistical accuracy.14,15
2. Assessment of clinical severity
The main outcome measures were assessed with several clinical scales which were categorized into two groups:
Patient-based questionnaires and physician-based quantita- tive scales.16 Patient questionnaire group consisted of Symptom
Severity Scale (SSS), Functional Status Scale (FSS), and faces pain scale. We used faces pain rating scale (0, 2, 4, 6, 8, 10) where 0=no pain and 10=the worst pain imaginable.
Faces pain scale was adopted on the ground that a majority of patients with CTS seek medical attention because of pain and this symptom makes patient being languid consequently results in poor quality of life. These three questionnaires were completed by patients’ own hands right after electro- physiologic study had been done. For prevention of influ- encing on response, patient was kept from hearing about results of electrophysiologic study until completing questionnaires.
Historical-Objective (Hi-Ob) scale was used for physician- based quantitative clinical scale.
3. Inclusion criteria
All subjects must have both clinical presentation and con- firmatory electrophysiologic findings consistent with CTS in at least one hand.
If there were subjective symptoms that suggest CTS but results of NCS were not compatible with CTS, we did not include patients’ data for analysis. Based on a careful his- tory taking, patients with previous surgical operation or trauma on the wrist, history of cervical radiculopathy, bra- chial plexopathy or polyneuropathy by any causes were excluded. Patients with diabetes mellitus were also excluded to be free from chance of superimposed diabetic peripheral neuropathy.
4. Statistical analysis
The statistical analyses were carried out by the SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Because data of each group did not show normal distribution, Kruskal-Wallis test was used to determine whether there was significant difference between groups for continuous variables including SSS and FSS. For a type of ordinal scales, such as faces pain scale and Hi-Ob scale, Chi-Square test was used to investigate correlation with electrophysiologic severity. Statistical sig- nificance was set up at p<0.05. Spearman correlation analy- sis was conducted for non-normal distributed continuous variables and ordinal variable to evaluate the degree of cor- relation between electrophysiologic severity and clinical
Table 1. General demographic characteristics
Electrophysiologic severity Age (years)a Number of patients (F/M) Symptom duration (months)b
Class 1 55.7±11.8 23 (19/4) 5.1±8.7 (0.3-36)
Class 2 53.9±9.0 123 (93/30) 10.1±12.6 (0.3-60)
Class 3 55.7±11.9 44 (30/14) 14.5±14.4 (0.5-48)
Class 4 56.1±16.4 10 (8/2) 16.2±14.1 (3-36)
Total 54.6±10.4 200 (150/50) 14.7±37.9
p-value NS NS Class 1 vs. 3<0.001*
Class 1 vs. 4<0.001* Kruskal-Wallis non-parametric test was used for analysis due to non-normal distribution of the data.
*Post-hoc analysis using Mann-Whitney test was conducted for comparison between each two groups and Bonferroni-adjusted p<0.05/6 was considered as statistically significant.
aAge was represented as mean±SD; bSymptom duration was represented as mean±SD with minimum and maximum value.
F, female; M, male; NS, non-significant.
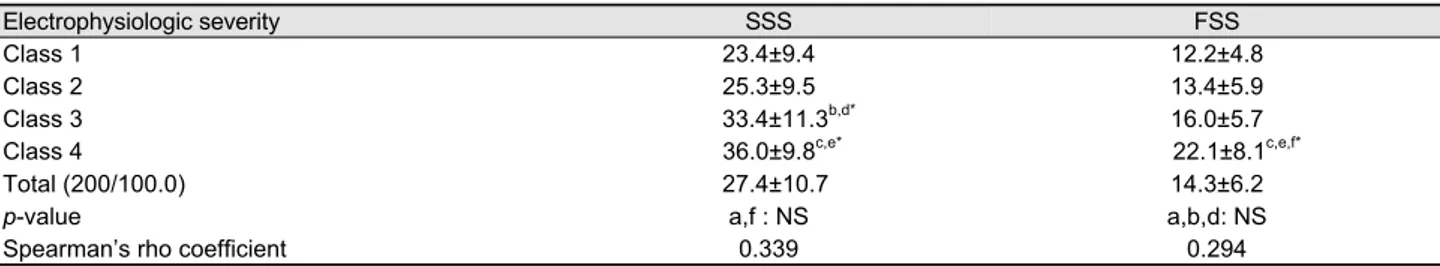
Table 2. Comparison of patient-based measurements according to elcetrophysiologic severity
Electrophysiologic severity SSS FSS
Class 1 23.4±9.4 12.2±4.8
Class 2 25.3±9.5 13.4±5.9
Class 3 33.4±11.3b,d* 16.0±5.7
Class 4 36.0±9.8c,e* 22.1±8.1c,e,f*
Total (200/100.0) 27.4±10.7 14.3±6.2
p-value a,f : NS a,b,d: NS
Spearman’s rho coefficient 0.339 0.294
Values are presented as mean±SD.
Kruskal-Wallis non-parametric test was used for analysis due to non-normal distribution of the data.
*Post-hoc analysis using Mann-Whitney test was conducted for comparison between each two groups and Bonferroni-adjusted p<0.05/6 was considered as statistically significant.
aClass 1 vs 2; bClass 1 vs 3; cClass 1 vs. 4, dClass 2 vs. 3; eClass 2 vs. 4; fClass 3 vs. 4; SSS, symptom severity scale.
FSS, functional status scale; NS, non-significant.
scales.
Results
Two hundred patients with documented CTS were finally enrolled (50 males, 150 females, mean age 54.6±10.4 years, 19-85). A median duration of disease was 5 months (1 week- 4 years). Thirty-three patients were affected by unilateral CTS and 167 were bilateral CTS.
According to the Padua scale, electrophysiologic severity was classified into six groups. As we enrolled patients based on abnormal electrophysiologic findings, no patient was classified into grade 0. Two patient (1.0%) were classified into minimal degree, 21 patients (10.5%) mild, 123 patients (61.5%) moderate, 44 patients (22%) severe and 10 patients (5.0%) extreme degree. As the number of each group was uneven and moderate group showed extremely large number of patients compared with other groups, we simplified the Padua scale into four categories: minimal and mild to class
1, moderate to class 2, severe to class 3, and extreme to class 4. Patients’ age and gender distribution were not statistically different between groups. In terms of symptom duration, pa- tients with more severe electrophysiologic degree showed longer disease duration. However, post-hoc analysis demon- strated statistically significant difference only between class 1 and class 3, class 4 (Table 1). The average score of SSS was 27.4±10.7 and there was statistically significant differ- ence between groups, but a weak correlation with the elec- trophysiological scale was shown (Spearman’s rho by sim- ple correlation analysis: 0.339, p<0.001). The average score of FSS was 14.3±6.2 and also showed statistically sig- nificant difference between groups, which also revealed a weak correlation with the electrophysiological scale (Spearman’s rho by simple correlation analysis: 0.294, p<0.001) (Table 2).
Based on the electrophysiologic scale, the distribution of pain scale demonstrated significant difference between groups (p=0.018). The majority of patients experienced mild pain (n=10, 43.5%) in class 1, and moderate pain
Figure 1. Distribution of pain scale demonstrated significant dif- ference between groups classified by modified Padua scale (p=0.018). A higher proportion of moderate to severe pain was found as electrophysiologic severity deteriorated.
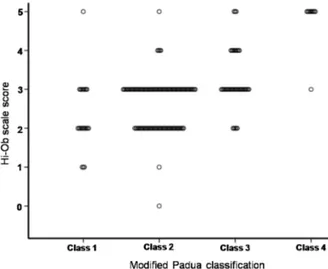
Figure 2. Scattered plot showed the moderate correlation be- tween electrophysiologic severity and physician-based clinical scale score (Spearman’s rho: 0.477, p<0.001). Hi-Ob, histor- ical-objective.
(n=42, 34.1%) in class 2. Moderate to severe level was de- tected in class 3 (n=22, 50%) or class 4 (n=5, 50%), re- spectively (Fig.1). However, pain intensity showed a weak correlation with electrophysiologic severity in our study (Spearman’s rho by Chi-square test=0.313, p<0.001). Physician- based clinical scales showed a moderate correlation with the electrophysiological scale (Spearman’s rho by Chi-square test: 0.477, p<0.001) (Fig.2).
Discussion
Severity of symptoms and functional status are the princi-
pal reasons that make patients visit clinics and seek proper management. So, value of clinical scales should be in- terpreted in view of how well it reflects patients’ status. In addition, considering that actual pathologic severity can be distinct from patients’ subjective symptoms, clinical scales should be well correlated with electrophysiologic findings.
That is, despite existence of typical symptoms suggesting CTS, nerve conduction studies can be normal. In previous study, 25% of the CTS patients had normal electro- physiologic findings and abnormal electrophysiologic find- ings were detected in older and heavier patients with more clear features of CTS.17 In such cases, there are several sup- positions explaining that CTS can have a local pathology limited to small unmyelinated fibers that the routine test cannot detect, or that the test done in the early stages of the disease can be normal.18 In our study design, we enrolled electrophysiologically demonstrated patients who have typ- ical symptoms and signs of CTS. In our opinion, although there are some possibilities of concealed CTS, the electro- physiologic abnormality is a fundamental precondition be- fore discussing about the severity. There is no universally accepted grading system for electrophysiologic severity and several grading scales are based on a number of established theories.7,19 In general, sensory fibers are more strongly af- fected than motor fibers,7,20 consequently resulting in an ear- lier reduction of a sensory nerve action potential amplitude than a compound muscle action potential.7,19,21 We selected the Padua scale, which were widely used in many studies, and modified grading system of Padua scale to compare with clinical scales. Among them, physician-measured quantitative scale was more correlative than patient-oriented symptom scales. This result is similar to that of a previous study in some ways.16 However, there are some difference between two studies. In our study, a total of 200 subjects were enrolled and all patients had both clinical symptoms and electrophysiologic abnormalities. In addition, simplified Padua scale was used for electrophysiologic severity grading.
Hi-Ob scale had been validated already as a reliable meas- urement,22 and was also proved as most correlative scale with electrophysiologic severity (p<0.001, rho=0.477) in our study. A moderate correlation between physician-based quantitative scales and electrodiagnostic severity suggests not only subjective symptoms descripted by patients, but also
physical findings such as thenar muscle atrophy and weakness examined thoroughly by physician are all important. Faces pain scale has been widely utilized as easy-to-use pain as- sessment tool even for child. We expect that it will also be useful for patients with intellectual disability, low education level or focal neurologic deficit who have difficulty in un- derstanding questionnaires. However, It is still debated whether pain is a typical feature of CTS, and some authors insist that pain without numbness is not a characteristic of the CTS but rather pain intensity can be predicted from ill- ness behavior (specifically depression and misinterpretation of nociception).4,23
This study has some limitations. The patients were en- rolled in a single hospital and moderate group was distinctly larger compared with other groups. Patient’s most bothering hand was not always concordant with the electrophysiologically worst hand. Therefore, they might answer based on clinical severity of the less affected hand in view of electrodiagnostic evaluation. In addition, the Korean language version of the scales requires validation in terms of its reliability, re- sponsiveness, and consistency. Recently, the Korean version of the Carpal Tunnel Questionnaire was published in July 2015 as a part of this effort.24 In conclusion, electrophysiologic se- verity of CTS was more correlated with physician-measured clinical scales than patient-oriented symptom scales.
Acknowledgements
This work was supported by the 2011 Inje University re- search grant.
REFERENCES
1. Roh YH, Chung MS, Baek GH, Lee YH, Rhee SH, Gong HS.
Incidence of clinically diagnosed and surgically treated carpal tunnel syndrome in korea. J Hand Surg 2010;35:1410-1417.
2. Atroshi I, Englund M, Turkiewicz A, Tägil M, Petersson I F.Incidence of physician-diagnosed carpal tunnel syndrome in the general population. Arch Intern Med 2011;171:943-944.
3. Aroori S, Spence RA. Carpal tunnel syndrome. Ulster Med J 2008;77:6-17.
4. Duckworth AD, Jenkins PJ, McEachan JE. Diagnosing carpal tunnel syndrome. J Hand Surg Am 2014;39:1403-1407.
5. LaJoie AS, McCabe SJ, Thomas B, Edgell SE. Determining the sensitivity and specificity of common diagnostic tests for carpal tunnel syndrome using latent class analysis. Plast Reconstr Surg
2005;116:502-507.
6. Chan L, Turner JA, Comstock BA, Levenson LM, Hollingworth W, Heagerty PJ, et al. The relationship between electro- diagnostic findings and patient symptoms and function in carpal tunnel syndrome. Arch Phys Med Rehabil 2007;88:19-24.
7. Longstaff L, Milner RH, O'Sullivan S, Fawcett P. Carpal tunnel syndrome: the correlation between outcome, symptoms and nerve conduction study findings. J Hand Surg Br 2001;26:475-480.
8. You H, Simmons Z, Freivalds A, Kothari MJ, Naidu SH.
Relationships between clinical symptom severity scales and nerve conduction measures in carpal tunnel syndrome. Muscle Nerve 1999;22:497-501.
9. Schrijver HM, Gerritsen AA, Strijers RL, Uitdehaag BM, Scholten RJ, de Vet HC, et al. Correlating nerve conduction studies and clinical outcome measures on carpal tunnel syndrome:
lessons from a randomized controlled trial. J Clin Neurophysiol 2005;22:216-221.
10. Dhong ES, Han SK, Lee BI, Kim WK. Correlation of electro- diagnostic findings with subjective symptoms in carpal tunnel syndrome. Ann Plast Surg 2000;45:127-131.
11. American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome:summary statement. Muscle Nerve 2002;25:918-922.
12. Baysal AI, Chang CW, Oh SJ. Temperature effects on nerve conduction studies in patients with carpal tunnel syndrome.
Acta Neurol Scand 1993;88:213-216.
13. Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand 1997;
96:211-217.
14. Padua L, Pasqualetti P, Rosenbaum R. One patient, two carpal tunnels: statistical and clinical analysis-by hand or by patient?
Clin Neurophysiol 2005;116:241-243.
15. Mondelli M, Filippou G, Gallo A, Frediani B. Diagnostic utility of ultrasonography versus nerve conduction studies in mild car- pal tunnel syndrome. Arthritis Rheum 2008;59:357-366.
16. Jung SH, Paik NJ, Bang MS, Han TR. Comparison of various clinical scales with electrophysiological scales for carpal tunnel syndrome. J Korean Assoc EMG Electrodiagn Med 2005;7:79-89.
17. Witt JC, Hentz JG, Stevens JC. Carpal tunnel syndrome with normal nerve conduction studies. Muscle Nerve 2004;29:515-522.
18. Wright SA, Liggett N. Nerve conduction studies as a routine di- agnostic aid in carpal tunnel syndrome. Rheumatology(Oxford) 2003;42:602-603.
19. Graham RA. Carpal tunnel syndrome: a statistical analysis of 214 cases. Orthopedics 1983;6:1283-1287.
20. Kimura I, Ayyar DR. The carpal tunnel syndrome: electro- physiological aspects of 639 symptomatic extremities. Electro- myogr Clin Neurophysiol 1985;25:151-164.
21. Stevens JC. AAEE minimonograph #26: The electrodiagnosis of carpal tunnel syndrome. Muscle Nerve 1987;10:99-113.
22. Giannini F, Cioni R, Mondelli M, Padua R, Gregori B, D'Amico P, et al. A new clinical scale of carpal tunnel syndrome: vali- dation of the measurement and clinical-neurophysiological assessment. Clin Neurophysiol 2002;113:71-77.
23. Nunez F, Vranceanu AM, Ring D. Determinants of pain in pa- tients with carpal tunnel syndrome. Clin Orthop Relat Res 2010;
468:3328-3332.
24. Kim JK, Lim HM. The Korean version of the Carpal Tunnel Questionnaire. Cross cultural adaptation, reliability, validity and responsiveness. J Hand Surg Eur Vol 2015;40:200-205.