251
ABBREVIATIONS: RA, retinoic acid; GFAP, glial fibrillary acidic protein.
Received May 28, 2009, Revised June 9, 2009, Accepted June 12, 2009
*Corresponding to: Jeong-Hwa Lee, Department of Biochemistry, College of Medicine, The Catholic University of Korea, 505, Banpo- dong, Seocho-gu, Seoul 137-701, Korea. (Tel) 82-2-2258-7293, (Fax) 82-2-596-4435, (E-mail) leejh@catholic.ac.kr
†
Co-Corresponding author: Ho-Joong Youn, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, 505, Banpo-dong, Seocho-gu, Seoul 137-701, Korea. (Tel) 82-2-3779-1066, (Fax) 82-2-780-3132, (E-mail) younhj@catholic.ac.kr
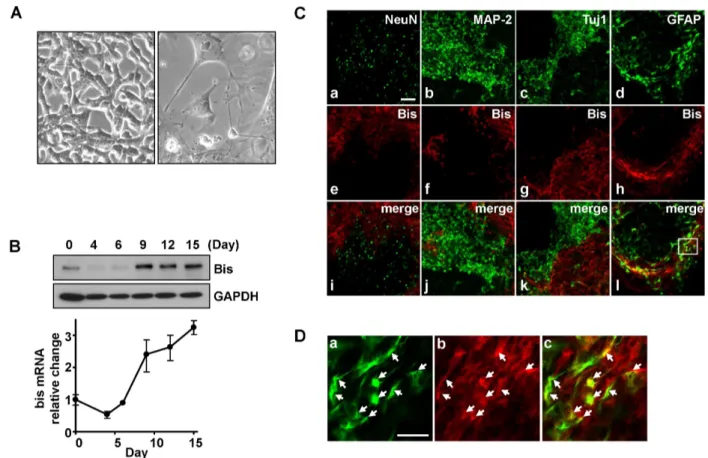
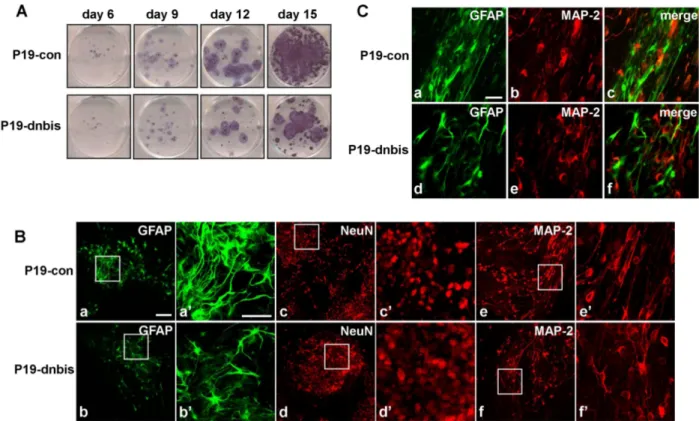
Bis Is Involved in Glial Differentiation of P19 Cells Induced by Retinoic Acid
Jung-Sook Yoon
1, Mun-Yong Lee
2, Jae-Seon Lee
3, Chan Sun Park
4, Ho-Joong Youn
5,*, and Jeong-Hwa Lee
6,†Departments of
1Biomedical Science,
2Anatomy, Graduate School, College of Medicine, The Catholic University of Korea, Seoul 137-701,
3