pISSN 1738-3544 eISSN 2288-1662
Analysis of Bone Mineral Density according to Hemoglobin in University Students
Joon Yoon 1 , Dai-Joong Kim 2 , Hyun-Ho Sung 3 , Yoon-Kyung Jo 3
1
Department of Radiologic Technology, Dongnam Health University, Suwon 16328, Korea
2
Department of Laboratory Medicine, Bundang Jesaeng Hospital, Seongnam 13590, Korea
3
Department of Clinical Laboratory Science, Dongnam Health University, Suwon 16328, Korea
혈색소 농도에 따른 대학생의 골밀도 분석
윤 준 1 , 김대중 2 , 성현호 3 , 조윤경 3
1
동남보건대학교 방사선과,
2분당제생병원 진단검사의학과,
3동남보건대학교 임상병리과
This study was performed to evaluate the effect of hemoglobin (Hb) on bone mineral density (BMD) in university students by performing a quantitative analysis. The subjects included healthy university students aged 20 to 30 years. Although osteoporosis has traditionally been considered as a disease of aging women, it is becoming an increasingly concerning male health problem.
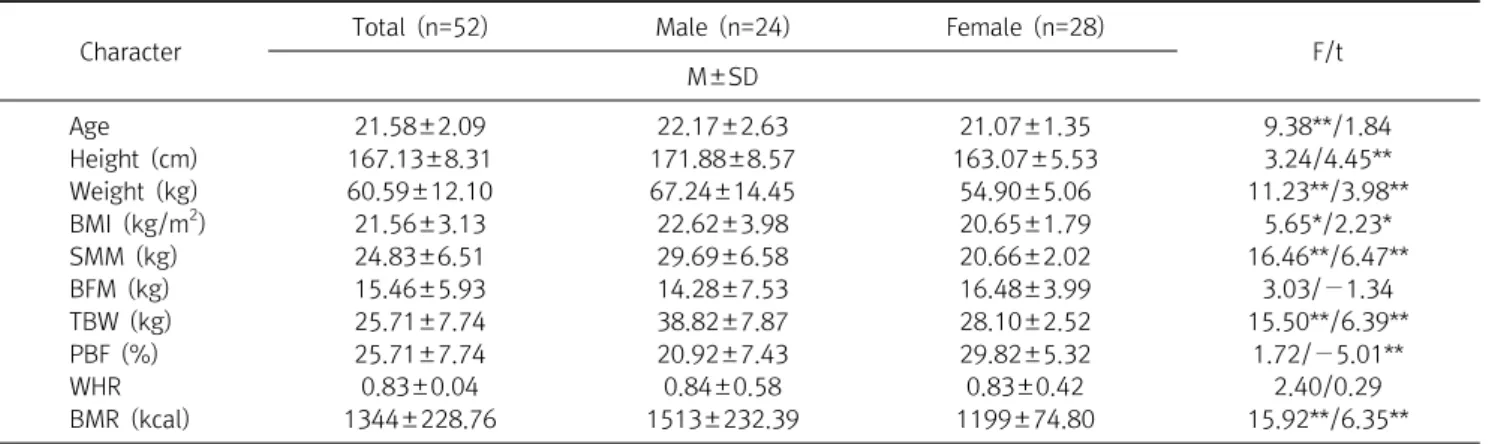
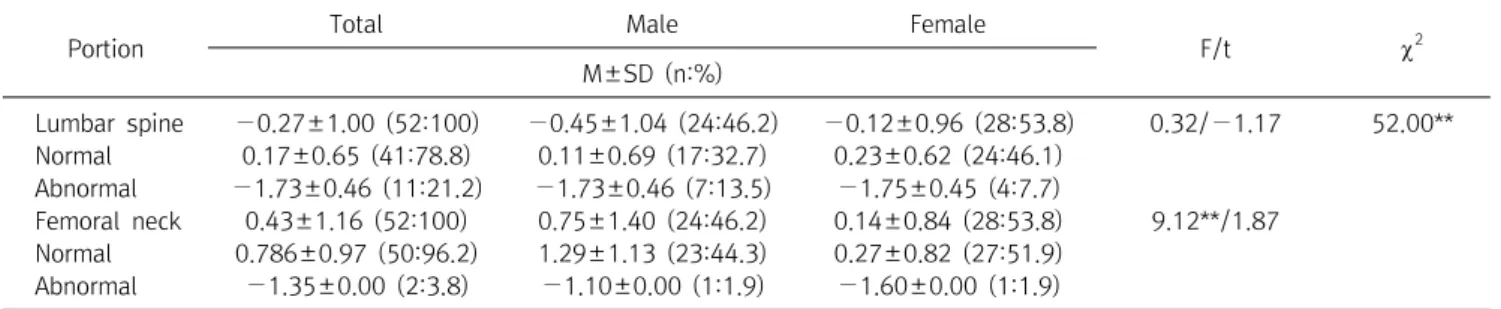
Diagnosis of osteoporosis is calculated with a quantitative assessment of BMD. Laboratory blood and urine tests are mainly used with low BMD or fragility fractures to identify any possible causes of bone metabolism disorders. In this study, there was no difference in BMD according to gender.
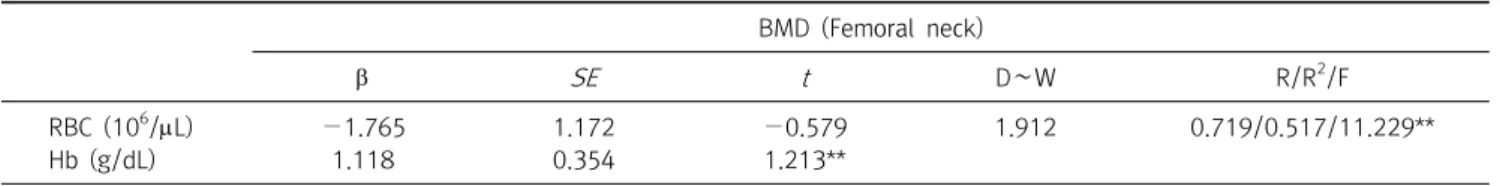
The average red blood cell (RBC), Hb, and Hematocrit (HCT) were significantly higher in males ( p <0.01). The correlation between lumbar spine, skeletal muscle mass (SMM), and basal metabolic rate (BMR) was statistically significant ( p <0.01). Hb showed a 51.7% statistical influence on BMD by multiple regression analysis. These findings are useful to understand the relationship between BMD and Hb; lower Hb level is associated with lower BMD. The Hb level was the strongest predictor of abnormal BMD. In conclusion, this study showed that a low Hb value was significantly correlated with low bone mass, suggesting that a low Hb value is a risk factor for changes in bone turnover that leads to a decrease bone density.
Key words: Bone mineral density, Hemoglobin
Corresponding author: Yoon-Kyung Jo Department of Clinical Laboratory Science, Dongnam Health University, 50 Cheoncheon-ro 74-gil, Jangan-gu, Suwon 16328, Korea
Tel: 82-31-249-6412 Fax: 82-31-249-6410 E-mail: ykjo@dongnam.ac.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Copyright © 2016 The Korean Society for Clinical Laboratory Science. All rights reserved.
Received: October 21, 2016 Revised: November 16, 2016 Accepted: November 17, 2016