저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Transient receptor potential vanilloid 1 Exhibits
Neuroprotective Effects in LPS-induced Dopaminergic
Neuronal Death in the Substantia Nigra in vivo
by
Eugene Bok
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
Transient receptor potential vanilloid
1
Exhibits
Neuroprotective Effects in LPS-induced Dopaminergic
Neuronal Death in the Substantia Nigra in vivo
by
Eugene Bok
A Dissertation Submitted to at the Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
Ph. D. in Neurosciences
Supervised by
Eun Joo Baik, M.D., Ph.D.,
Byung Kwan Jin, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Eugene Bok is approved.
SUPERVISORY COMMITTEE
Hyuk Wan Ko
Eun Joo Baik
Yong Beom Lee
Eun Hye Joe
Byung Kwan Jin
The Graduate School, Ajou University
December, 23rd, 2010
ACKNOWLEDGEMENT
생명체를 연구하는 것은 너무나 신나는 일입니다. 어제 먹은 요구르트의 유산균부터 오스트레일리아의 샤크만에 있는 스트로마톨라이트까지, 35억년 전의 지구를 기억하는 박테리아를 지금 이 순간 느낄 수 있다는 것은 흥미롭습니다. 단세포 박테리아가 자신의 최대 무기인 불멸성을 버리고 진핵 생물로의 진화를 통해 얻은 복합적이고도 정교하고도 아름답고도 그 어떤 기계보다도 높은 효율성을 지닌 훌륭한 생체 시스템을 관찰하는 것 은 더욱 흥미롭고요. 우주의 탄생 과정에서 기적적으로 생겨난 생명체, 특히 고등 생명체 의 경우 그 탄생 과정도 멋지지만, 뇌세포와 심장의 근육 세포와 온몸을 돌아다니는 혈 액세포와 저 발끝의 피부세포가 그들 각자의 수명과는 상관 없이 개체의 수명에 자신들 의 운명을 맡기고 한꺼번에 소멸해가는 죽음마저도 얼마나 우아한지, 무엇 하나 신기하 지 않은 부분이 없는 듯 합니다. 그 중에서도 지금의 나를 나로 인식하게 하고, 상상 이 상의 정교한 작업을 가능하게 해주는 신경계는 더할 나위 없이 재미있는 분야이고, 학위 과정 동안 이런 매력적인 대상을 연구할 수 있었던 것은 제 인생에 가장 큰 행운이었습 니다. 좋은 일, 나쁜 일, 기쁜 일, 슬픈 일 등이 공존했던 그 행운의 시간, 다양한 이벤트 로 빽빽하게 연결된 스펙트럼과도 같았던 지난 5년, 저의 마음과 머리를 채워주며 그 시 간이 헛되지 않도록 도와준 많은 분들께 이 지면을 통해 감사 드립니다. 우선 평생의 스승님이신 진병관 교수님, 과학자로서, 인생 선배님으로서 보여주신 가르 침을 통해 학자로서의 역량뿐만 아니라 사람 사이의 예절 등, 많은 부분을 배우고 성장 할 수 있었습니다. 언제나 아낌없는 조언과 함께 철학적인 질문을 해주시는 이용범 교수 님, 연구자로서 안일함에 빠지지 않도록 이끌어 주셨던 조은혜 교수님, 프리즌테이션부터 논문의 퇴고까지 많은 신경을 써 주신 고혁완 교수님 고맙습니다. 학교를 옮기는 과정에 서 많은 배려를 해주신 백은주 교수님, 교수님의 배려가 없었다면 지금 이 순간은 오지 않았을 것입니다. 감사합니다. 같은 길을 가고 있는 우리 실험실 멤버들에게도 고마움을 전합니다. 앞서서 저를 잘 이끌어준 영철 오빠, 존재만으로 위안이 되는 수희 언니, 늦은 나이에도 언제나 열정적으 로 사는 은수 오빠, 우리 랩의 희망 진한이. 앞만 보고 달려왔는데, 정작 앞은 보이지 않 았던 두렵고 답답했던 시간들, 함께 걸어가는 동료들이 있다는 것은 큰 위안이었습니다.랩 사람들과 교수님 사이에서 균형을 맞추려고 노력하던 민영이와 졸업하고 떠난 상룡 오빠, 은숙 언니, 근우 오빠, 소윤 언니도 고맙습니다. 그리고 일일이 열거할 수 없는 아 주대, 경희대 대학원 선후배님들, 자랑스런 동기분들, 여러분들과 함께 했던 많은 과학적 토론과 일상의 잡다한 수다는 언제나 즐거웠습니다. 그리고 학위 과정 내내 행정적인 문 제로 귀찮게 해드렸는데도 문제가 생기지 않도록 항상 잘 처리해주셨던 대학원 행정실의 이영옥 선생님께도 감사드립니다. 항상 같은 자리에서 지켜봐 주고 응원해 주는 해자 클럽, 목련반, 누님 패밀리 친구들. 특히, 엄마 같은 눌, 윤선, 이제는 진짜 엄마가 된 지영 언니, 청춘의 방황을 공유하던 껍. 철 없는 대학원생의 치기와 무심함과 빈곤함마저 이해하고 감싸주었던 나의 멋진 친구들, 평생 잊지 않을게요. 자식들의 꿈을 위해 평생 투자만 하고 계시는 부모님, 부모님의 신뢰가 제가 살아가는 데 가장 큰 힘이 되었습니다. 꿈꾸는 청년인 오빠, 같이 영화며, 책이며, 우주의 삼라만상 까지 새벽 내내 흥분해서 떠들어 댔던 그 순간들이 난 항상 즐거웠어. 언제나 제 편이 되어주고 힘들 때마다 가장 가까운 곳에서 용기와 위안을 주었던 가족들 감사하고 사랑 합니다. 아직도 생물에 대한 궁금증은 너무나 많습니다. 면역학적으로 난자는 왜 정자를 외부 물질로 인식하지 않는지, 왜 어제 반복해서 읽었던 논문의 내용이 랩미팅 시간에 교수님 이 질문만 하면 머리 속에서 사라지는지, 기타를 칠 때, 나의 손가락은 왜 드림씨어터의 ‘존 페트루치’처럼 움직이지 않는지, 백날 연구하고 있는 암은 언제쯤 정복할 수 있는지 등등. 앞으로 저는 그 중에서도 가장 궁금한 뇌 과학의 세계, 1300g의 용량을 가진 20W 의 어두침침한 세계 속에서 스스로 질문을 던지고 해답을 찾아가며 꿈꾸는 탐험가로서의 삶을 지속할 생각입니다. 아직 부족한 점은 많지만 무사히 졸업을 하고 소중한 이 학위 논문이 나오기까지 도움 주셨던 많은 분들께 다시 한번 감사드립니다.
i
ABSTRACT
-Transient receptor potential vanilloid 1 Exhibits Neuroprotective
Effects in LPS-induced Dopaminergic Neuronal Death in the
Substantia Nigra in vivo
Parkinson’s disease (PD) is a motor disorder which is progressive degeneration of the nigrostriatal dopaminergic pathway. There is no cure for PD and besides the etiology of this disease was limited understood. Neuroinflammation have been proposed to play a role in the pathogenesis of PD. Recent evidence implicates the transient receptor potential vanilloid subtype 1 (TRPV1) leads to neuroprotective effects by anti-inflammtory action. The main purpose of this thesis is to investigate the functional effects of capsaicin (CAP), the TRPV1 agonist, in LPS-induced in vivo PD model. To do that, we investigated the LPS-induced inflammatory response including dopaminergic (DA) neuronal degeneration, microglia activation, inflammatory mediator expression and infiltration of peripheral immune cells in the SN. Additionally, we surveyed whether CAP would have ability to DA neuronal protection against LPS and how CAP regulates the inflamed condition. We also confirmed whether these effects of CAP were mediated by TRPV1. Immunostaining, RT-PCR and western blot analysis showed that CAP attenuates LPS-induced production of proinflammatory mediators such as inducible nitric oxide synthase (iNOS) and ROS while CAP increases Arginase 1 and CD206, M2 macrophage markers. In addition, CAP protected nigral DA neurons from LPS-induced neurotoxicity. This neuroprotective effect was reversed
ii
by capsazepine, a TRPV1 antagonist, indicative of TRPV1 involvement. However CAP could not block the infiltration of peripheral immune cells. Based on these results, we found that CAP exhibits the neuroprotective effects through regulating the immune cell phenotypes. The present results suggest that CAP may be beneficial for the treatment of neurodegenerative diseases, such as PD that is associated with neuroinflammation via TRPV1.
iii
TABLE OF CONTENTS
ABSTRACT ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ i TABLE OF CONTENTS ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ iii LIST OF FIGURES ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ vi ABBREVIATION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ vii I. INTRODUCTION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 1 A. Parkinson's disease ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 1 1. The characteristics of PD ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 1 2. Inflammation in PD ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 2 3. LPS model of PD ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 5 B. Immune cells in CNS ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 7 1. The characteristics of microglia ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 7 2. Infiltration of peripheral immune cells in CNS ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 9 3. Polarization of the immune cells ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 11 4. iNOS versus Arginase 1 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 13 C. Capsaicin and their receptor TRPV1 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 14 1. Capsaicin ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 14 2. Transient receptor potential vanilloid subtype 1 (TRPV1) ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 15 3. The neuroprotective effects of CAP and TRPV1∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 16 D. Aims of this study ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 17 II. MATERIALS AND METHODS ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 18
iv
A. Materials and methods ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 18 1. Stereotaxic surgery ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 18 2. Pharmacological treatments ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 18 3. Tissue preparation and immunohistochemistry ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 19 4. Double-immunofluorescence Staining ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 21 5. Stereological cell counts of DA neurons ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 23 6. Counts of immuno-positive cells ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 23 7. Polymerase chain reaction (RT–PCR) ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 23 8. Western blot analysis ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 24 9. In situ detection of O2- production ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 26
10. Cortical and mesencephalic microglia cultures ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 26 11. Determination of NO ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 27 12. Statistical analysis ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 27 III. RESULTS ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 30 A. The features of intranigral injection of LPS in the SN ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 30 1. LPS induces degeneration of DA neurons and activation of microglia in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 30 2. LPS induces activation of microglia in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 34 3. LPS induces proinflammatory cytokines, iNOS and COX-2 in CD11b-ip cells in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 36 4. LPS induces infiltration of various peripheral immune cells in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 39 B. The neuroprotective effects of CAP ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 42
v
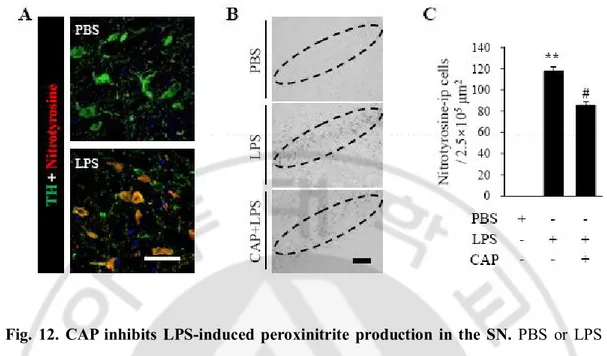
1. CAP protects DA neurons from LPS-induced neurotoxicity via TRPV1 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 42 2. CAP fails to undergo quantitative change of LPS-induced peripheral immune cells infiltration in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 45 3. CAP inhibits LPS-induced mRNA production of IL-1β and iNOS in the SN. ∙∙∙∙∙∙∙ 48 4. CAP oppositely regulates M1 and M2 macrophages marker expression in the SN.
∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 50 5. iNOS or Arginase 1 expressed in infiltrated immune cells. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 57 6. CAP inhibits LPS-induced iNOS expression and peroxinitrite production
in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 61 7. CAP reduces LPS-induced oxidative stress in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 63 8. CAP reduced NO release on mesencephalic microglia. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 66 IV. DISCUSSION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 69 A. The features of intranigral injection of LPS in the SN ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 69 B. The neuroprotective effects of CAP ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 71 V. CONCLUSION ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 79 REFERENCES ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 80 국문요약 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 107
vi
LIST OF FIGURES
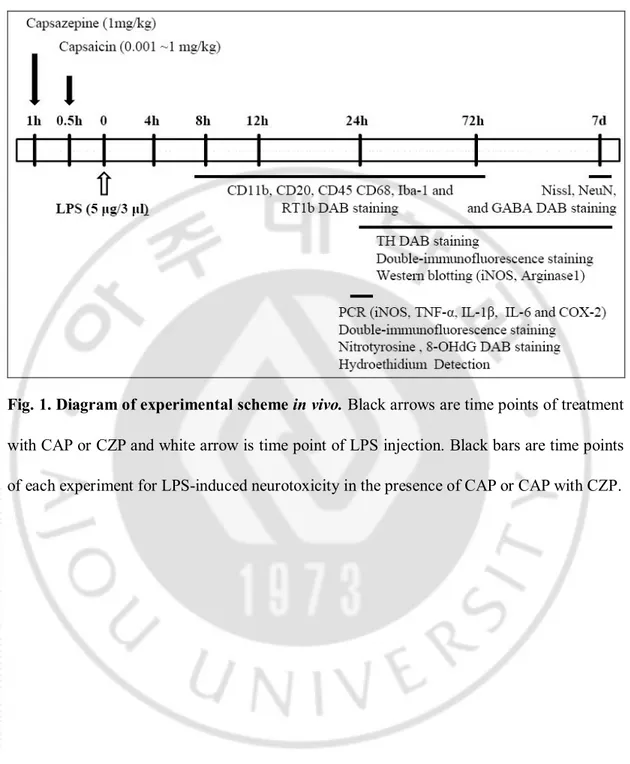
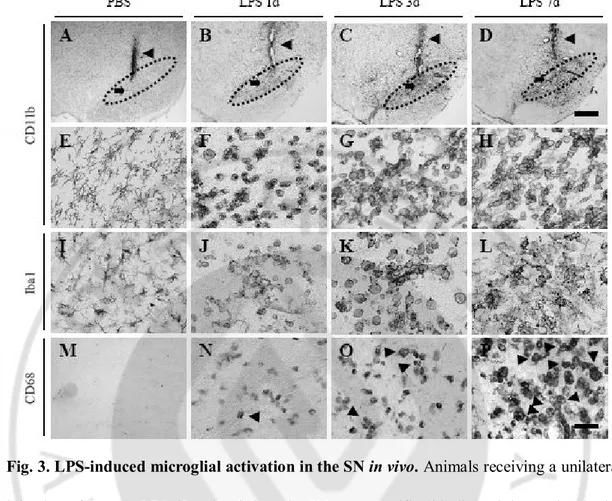
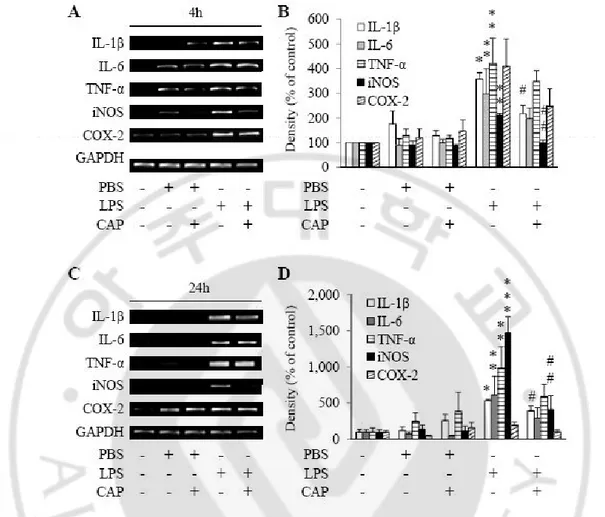
Fig. 1. Diagram of experimental scheme in vivo. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 29 Fig. 2. LPS-induced neurotoxicity on DA neurons in the SN in vivo. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 32 Fig. 3. LPS-induced microglial activation in the SN in vivo. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 35 Fig. 4. The expression of proinflammatory mediators in LPS-induced activated
microglia. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 37 Fig. 5. LPS-induced infiltration of peripheral immune cells in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 40 Fig. 6. The protection effects of CAP on LPS-induced neurotoxicity in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙ 43 Fig. 7. CAP fails to undergo quantitative change of LPS-induced peripheral immune
cells infiltration in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 46 Fig. 8. CAP inhibits LPS-induced mRNA production of IL-1β and iNOS in the SN. ∙∙∙∙∙∙∙∙∙ 49 Fig. 9.CAP oppositely regulates iNOS and Arginase 1 expression in CD11b-ip cells in
the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 52 Fig. 10. The effects of CAP on LPS-induces changes in the expression of proteins Associate with M1 and M2 macrophages ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 55 Fig. 11.iNOS or Arginase 1 expressed in infiltrated immune cells.∙∙∙∙∙ ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 59 Fig. 12. CAP inhibits LPS-induced iNOS expression and peroxinitrate production in
the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 62 Fig. 13. CAP reduces LPS-induced oxidative stress in the SN. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 64 Fig. 14. CAP reduced NO release on mesencephalic microglia. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 67
vii
ABBREVIATION
6-OHDA, 6-hydroxydopamine (6-OHDA) AD, Alzheimer's disease
ALS, amyotrophic lateral sclerosis AP, activator protein
BBB, blood brain barrier CAP, capsaicin
COX-2, cyclooxygenase-2
CMF-HBSS, Ca2+, Mg2+ free Hanks' balanced salt solution
CNS, central nervous system CSF, cerebrospinal fluid CZP, capsazepine DA, dopaminergic
DMEM, Dulbecco's modified Eagle's medium FBS, fetal bovine serum
GABA, gamma-aminobutyric acid
GAPDH, glyceraldehydes-3-phosphate dehydrogenase G-CSF, granulocyte colony-stimulating factor
Iba-1, ionized calcium binding adaptor molecule 1 IFN--γ, interferon-gamma
viii iNOS, inducible nitric oxide
ip, immuno-positive i.p., intraperitoneal LB, lewy bodies
MAPK, mitogen-activated protein kinase MHC, major histocompatibility complex MPO, myeloperoxidase
MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine NF-κB, nuclear factor-κB
PB, phosphate buffer
PBS, phosphate buffed saline PD, Parkinson's disease RNS, reactive nitrogen species ROS, reactive oxygen species RT, room temperature
RT-PCR, reverse-transcriptase polymerase chain reaction SNpc, substantia nigra pars compacta
SNr, substantia nigra reticulate
STAT, signal transducer and activator of transcription STR, striatum
SD, Sprague–Dawley
ix TGF- β, tansforming growth factor-β
TH, tyrosine hydroxylase TLR, toll-like receptor
TNF-α, tumor necrosis factor-alpha
TRPV1, transient receptor potential vanilloid subtype 1 (TRPV1) VTA, ventral tegmental area
1
I. INTRODUCTION
A. Parkinson's disease
1. The characteristics of PD
Idiopathic parkinsonism, or Parkinson's disease (PD), was first description by British physician Dr. James Parkinson in 1817 (Parkinson, 2002), is second most common neurodegenerative disorder after Alzheimer's disease (AD). It is primarily affecting people of ages over 55 years, although young adults and even children can also be affected. The incidence of PD is related to age, affects approximately 0.5 to 1 percent among persons 65 to 69 years of age, rising to 1 to 3 percent among persons 80 years of age and older (Nussbaum and Ellis, 2003). According to the United Nation’s report, the number of people aged over 60 will increase within the next 50 years, from 11 % of the total population in 2007 to 22% in 2050, nevertheless there is no cure for PD. L-DOPA treatment still remains the standard of PD therapies but long-term treated L-DOPA results in dyskinesia. Thus it is absolutely imperative that we should find the cause and treatments of this disease.
The neuropathological feature of PD is the degeneration of dopaminergic (DA) neurons of the substantia nigra pars compacta (SNpc) in the midbrain, and loss of their ascending projection to the striatum (STR).Progressive degeneration of the nigrostriatal dopaminergic pathway results in control of voluntary movements and these clinical signs of PD appear when striatal dopamine is reduced by approximately 80% (Savitt et al., 2006). PD related neuronal degeneration also occurs in the other brain regions including motor nucleus of the
2
glossopharyngeal and vagal nerves, anterior olfactory nucleus, anteromedial temporal mesocortex, and neocortex succumbs (Braak et al., 2003).
For these reasons, PD patients are primarily affected in brain areas which are involved in motor control. As the disease progresses, bradykinesia, a slight rhythmic tremor at rest, gait disturbances, postural instability and rigidity are developed. During the early or late in this disease, most PD patients suffered non-motor signs and symptoms such as olfactory dysfunction, fatigue, pain, autonomic dysfunctions, mood and sleep disorders and etc. (Fahn, 2003; Wolters, 2008).
Other neuropathological hallmark of PD is the formation of eosinophilic Lewy bodies (LBs) and Lewy neuritis in surviving DA neurons (Spillantini et al., 1997; Shults, 2006; Iwatsubo, 2007) which were first described by Frederich Lewy in 1912 (Gibb and Poewe, 1986). LBs found in other diseases, such as dementia with LBs, diffuse LB disease and AD and various brain lesions not only DA neuronal systems but also the noradrenergic (locus coeruleus), serotonergic (raphe), and cholinergic system (Braak et al., 2003). LBs in PD and Parkinsonism contain α-synuclein (Greenfield and Bosanquet, 1953; Spillantini et al., 1997), ubiquitin (Lowe et al., 1988) and other proteins (Robinson, 2008). These protein aggregation can disrupt normal cellular architecture that cell function is compromised, eventually leading to cell death (Shults, 2006).
2. Inflammation in PD
The underlying pathology of PD is well explored, but we have limited understanding of the etiology. Many studies, molecular cloning, biochemical characterization, pathology
3
analysis, and epidemiologic studies, have shown that the most (>95%) PD cases are sporadic occurrence characterized by late onset (Tanner, 2003) and small faction (<5%) of cases are familial clusters characterized by early onset (Mizuno et al., 2001). The familial PD may be caused by inherited mutations in several genes (for e.g. a-synuclein, Parkin, PINK, DJ-1, Leucine-rich repeat kinase-2, Ubiquitin carboxy-terminal hydrolase L1, ATP13A2 and etc.), some of which give rise to the same phenotype but with a different mode of inheritance. A genetic variant can be a mutation or a single nucleotide polymorphism (the latter is present at a frequency of >1% in the population), a deletion, an insertion, a whole gene rearrangement or a copy number variation (Bonifati, 2006; Westerlund et al., 2010). These mutants manifested the accumulation and aggregation associated LBs formation in PD patients.
In the case of sporadic PD, the cause of this has been subject to dramatic change in recent years. Meanwhile, evidence from human (McGeer et al., 1988; Langston et al., 1999) and animal studies (Kreutzberg, 1996; Liberatore et al., 1999; Gao et al., 2002) has suggested that the neuroinflammation plays an important role in the pathogenesis of PD. It was first reported by McGeer and his colleagues in 1998 from a post-mortem study. They found activated microglia and T-lympohcytes in the SNpc of a PD patient (McGeer et al., 1988). While increased levels of activated microglia is exacerbated in PD and they can released the neurotoxic factors. Therefore nigral neurons are under stress or damaged in PD, the DA neurons of the SNpc are degenerated. Consistent with these results, we reported that microglial activation involving the degeneration of DA neurons was observed in the SNpc (Choi et al., 2003; Kim et al., 2010).
4
Since then, numerous studies supported a role for neuroinflammatory processes in PD and also detected reactive oxygen species (ROS), pro-inflammatory cytokines, inducible nitric oxide (iNOS) and cyclooxygenase-2 (COX-2) in the post-mortem PD brain (Hunot et al., 1996; Hirsch et al., 1998; Knott et al., 2000; McGeer and McGeer, 2004; Whitton, 2007). Basal levels of lipid peroxidation are increased in the SN of PD patients (Dexter et al., 1989), and this area of brain is vulnerable to excess free radicals and ROS which may be produced by activated microglia as part of an inflammatory response.Oxidative stress is caused by an imbalance between the production and destruction of ROS and reactive nitrogen species (RNS) (Tieu et al., 2003). These include elevated oxidative damage to DNA, proteins and lipids, decreased levels of reduced glutathione and increased SOD and monoamine oxidase activity in the SNpc of PD (Hasegawa et al., 1990). ROS/RNS production could be modulated by microglial- NADPH oxidase and NOS in a neuropathological condition.In the MPTP model of PD, NADPH oxidase and iNOS are major sources of ROS/RNS production where they mediate DA neuronal death in the SN (Liberatore et al., 1999; Wu et al., 2006). Other in vivo and in vitro studies have revealed that microglia-derived NADPH oxidase and iNOS play critical roles in the generation of ROS/RNS and contribute to DA neuronal death via oxidative stress (Gao et al., 2002; Arimoto and Bing, 2003; Qin et al., 2005; Rodriguez-Pallares et al., 2007). This is consistent with our recent results that minocycline inhibits NADPH oxidase-/iNOS-derived ROS production through the activation of microglia, which prevents oxidative damage to neurons and leads to increased neuronal survival in thrombin-injected SN (Choi et al., 2005b). Proinflammatory substances such as COX-2 and proinflammatory cytokines, including interleukine-1beta (IL-1β), interferon-gamma (IFN-γ)
5
and tumor necrosis factor-alpha (TNF-α), have been elevated in the postmortem PD brain as well as in serum and cerebrospinal fluid (Mogi et al., 1994; Hunot et al., 1996; Mogi et al., 1996; Knott et al., 2000; Le et al., 2001). Similarly to PD patients, a 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD experienced increased levels of these inflammatory mediators as well as deteriorated DA neuronal death (Klevenyi et al., 1999; Ferger et al., 2004; Pott Godoy et al., 2008). In agreement with these findings, our recent results report that mitogen-activated protein kinase (MAPK) inhibitors spare nigral DA neurons through inhibition of thrombin- or prothrombin kringle-2-induced microglial activation as well as through the overexpression of proinflammatory cytokines in microglia (Choi et al., 2003; Kim et al., 2010). Additionally, induction of chronic expression of IL-1β in adult rat SNpc using a recombinant adenovirus resulted in DA neuronal death after three weeks (Ferrari et al., 2006). Taken together, these findings indicate that microglial activation-derived oxidative stress and proinflammatory molecules are deleterious to DA neurons in the SN.
3. LPS model of PD
Lipopolysaccharide (LPS) is an endotoxin found in the outer membrane of gram-negative bacteria. It is composed of three components which are O-antigen with multiple repeating units of monosaccharides, a polysaccharide core with an unusual sugar (2-keto-3-deoxyoctonate), and lipid A consisting of a unique diglucosamine backbone to which six fatty acid chains are attached. LPS associates with the soluble LPS binding protein and CD14, which interact of with the transmembrane Toll-like receptor-4 (TLR-4) and the
6
extracellular accessory protein. This association leads to the activation of kinases of various intracellular signaling pathways and upregulation of gene transcription for a variety of proinflammatory factors and free radical-generating enzymes. For such reasons, LPS can stimulate both peripheral immune cells (macrophages and monocytes) and central nervous system (CNS) glia (microglia and astrocytes) and causes their release of various immunoregulatory and proinflammatory cytokines and free radicals (Raetz et al., 1991; Dentener et al., 1993; Medvedev et al., 2000). In contrast, LPS does not have a direct effect on neurons because of their lack of functional expression of TLR-4 (Lehnardt et al., 2003). Nevertheless LPS is not classical toxin for PD models, it is the suitable tool to study inflammation-mediated DA neurodegeneration.
There are various LPS-induced PD animal models. A single injection of LPS to the SN region of Wistar, Fisher or Sprague–Dawley (SD) rats leads to a significant loss (50–85%) of SNpc DA neurons (Castano et al., 1998; Lu et al., 2000). Unilateral SN injection of 2 μg LPS to Wistar rats decreased DA levels in both the striatum and SN (∼50%) up to 21 days after LPS injection (Castano et al., 1998). Chronic infusion of LPS using osmotic mini-pump to the SN induced microglia activation and DA neuronal degeneration. Furthermore intraperitoneal injection (i.p.) of 5 mg/kg of LPS in C57 mice has been indicated to influence the activities of the immune cells in the brain and consequently contributes to the chronic neurodegenerative process reached 23% and 43% at 7 and 10 months, respectively (Perry, 2004). In the LPS models above mentioned, microglial activation was detected and TNF-α and IL-1β were increased in the SN. These pro-inflammatory cytokines are thought to be involved in LPS-mediated toxicity (Gayle et al., 2002). Besides, the C57BL/6 mice which
7
received five injections of LPS (5 mg/kg, i.p.) at monthly intervals shown progressive loss of DA neurons in the SN and significant differences in behavior, measured by rotor-rod and open-field activities (Liu et al., 2008).
B. Immune cells in CNS
1. The characteristics of microglia
Microglia are the resident immune cells of the CNS and constitute about 5-20% of all glial cells. They were first identified as a distinct cell entity by Pio del Rio-Hortega in 1930s (Lawson et al., 1990). The origin of microglia is one of the most controversial issues in glial research.There are three main hypotheses in regard to the origin of microglia. One of them is that microglia are arisen mesohaemopietic cells from myeloid tissue (Cuadros and Navascues, 1998). Another concept of origin of microglia is circulating blood monocytes invade the brain and became the progenitor of microglia. However microglial progenitors unfortunately have been demonstrated in situ in the embryonic brain before the circulatory system has developed (Morris et al., 1991). Lastly it has been suggested that microglial progenitors are derived from neuroectoderm. The resident microglia in the CNS can self-renew and undergoes mitosis to increase the numbers of microglia in the affected area during insult (Ajami et al., 2007). In conclusions, the numerous neurobiologists have been postulated that microglia derived from blood monocytes post-natally and during development microglial precursors enter the CNS via different routes such as from the meninges, ventricular lumen or the blood stream. Precursors migrate within the nervous parenchyma to their final location. These microglial cells show features of ameboid form
8
while they move through the nervous parenchyma, but differentiate and become mature ramified form once they reach their final location (Cuadros and Navascues, 1998; Kaur et al., 2001).
In the healthy brain, resting microglia adopt a ramified morphology consisting of small cell bodies and many slim branchings, containing up to 20 spines (Kreutzberg, 1996). Little is known about the functions of resting microglia, but they are suggested to function in immune surveillance and host defense by releasing low levels of growth factors to support the surrounding neurons (Nimmerjahn et al., 2005). Activated microglia also occurs in healthy brain during development and remodelling. They phagocytize the neurons and cellular debris to prevent excessive cell production (Ashwell, 1991). During embryonic and early post-natal development of the brain, they supported to the successful synaptic connections of neurons by producing of growth factors (Batchelor et al., 1999).
Resting microglia are rapidly activated by various stimulators such as changes in the extracellular environment of the injured or diseased brain and characterized morphological changes from a resting, ramified shape to an active amoeboid shape to facilitate proliferation, migration and phagocytosis (Nimmerjahn et al., 2005). Several studies have revealed that activated microglia express diverse cell-surface receptors, including the major histocompatibility complex (MHC) and complement receptors (Graeber et al., 1988; Kreutzberg, 1996) as well as inducible potentially neurotoxic factors (Kim and de Vellis, 2005) that impose chronic inflammation of the brain, leading to neuronal dysfunction and death in the form of neurodegenerative disorders such as PD (Orr et al., 2002).
9
and in vitro (Kim and de Vellis, 2005). Activated microglia have the ability to produce cytokines, chemokines, neurotrophines, ROS and RNS (Garden and Moller, 2006). Cytokines, low molecular-weight proteins, are usually classified as pro- or anti-inflammatory and play an important role in various physiological processes both by paracrine and autocrine methods. Generally pro-inflammatory cytokines induce the immune response while anti-inflammatory cytokines act to down-regulate an immune response (Hanisch et al., 2004).
2. Infiltration of peripheral immune cells in CNS
CNS has been considered an immunologically-privileged region due to the presence of innate microglia. Actually under normal conditions, the CNS is restricted by blood brain barrier (BBB) and cerebrospinal fluid (CSF) from many toxic substances.
BBB are
comprised of neurovascular units such as brain capillary endothelial cells, pericytes,
and astrocyte endfeet. Endothelial cells connect as tight junctions, thereby conferring
low paracellular
permeability. Pericytes and astrocytes regulate hemodynamic
neurovascular coupling,
microvascular permeability, matrix interactions,
neurotransmitter inactivation, neurotrophic
coupling, and angiogenic and neurogenic
coupling through close proximity with neurons
(Zlokovic, 2008).
Although there is no
clear evidence whether BBB leakage is related the loss of DA neurons in PD, our
recent studies on
animal models suggest a pathogenic linkage between BBB
10
combined with infiltration of peripheral immune cells important roles in the
degeneration of DA neurons (Chung et al., 2010).
The neuroinflammation may contribute to the infiltration of peripheral immune
cells and leakage
of the BBB in the SN.
Actually in the midbrain ofpostmortem PD
patients, CD4 and CD8 positive cells were numerous detected. This is suggested the
potential role
of infiltrated peripheral cells is related to PD pathogenesis (McGeer et al., 1988; Brochard et al., 2009). Recent several studies shown that blood derived various peripheral immune cells can enter the CNS. The infiltrated CD4+ lymphocytes contribute to the neurodegeneration in MPTP PD model (Appel, 2009) and recruited CD8+ lymphocytes exacerbate or even to initiate CNS neuropathology (Carson et al., 1999). In LPS injected rat models, neutrophils and monocytes infiltrate to the SN, cortex and hippocampus regions, lead to the neuroinflammation (Ji et al., 2007). These results suggest that penetration of immune cells plays an important role in the degeneration of DA neurons in PD.On the other hand, several studies supported that infiltrating cells also can induce beneficial effects. In AD animal models, infiltrating monocytes and macrophages restricted amyloid deposit (Simard et al., 2006; Town et al., 2008; Hawkes and McLaurin, 2009). Either CD4+ T lymphocyte can serve to limit neuronal loss through by shifting the trophic/cytotoxic balance of glia in ALS model (Beers et al., 2008; Chiu et al., 2008). Additionally infiltrating leukocytes is a critical host defense mechanism against West Nile virus in encephalitis (Town et al., 2009).
11
These results suggested that infiltration of peripheral immune cells may affect beneficial or deleterious impacts in certain circumstances (Rezai-Zadeh et al., 2009; Gate et al., 2010).
3. Polarization of the immune cells
Microglial activation is major component of the CNS pathology. Until recently, there was controversial evidence as to whether activated microglia promoted neuronal survival or exacerbated neuronal death. In studies of neuronal injury models, microglia activation may have abilities to either promote neuronal protection or injury. Microglia and macrophage display functional plasticity and can change their physiology during activation, involving changes in cell number, morphology, surface receptor expression, and production of growth factors and cytokines (Gordon and Taylor, 2005; Benoit et al., 2008; Mosser and Edwards, 2008).
Polarized macrophages have been classified into two group, M1 and M2 macrophages (Benoit et al., 2008; Mosser and Edwards, 2008). Classically activated M1 pro-inflammatory macrophages were described in 1970s. They can be induced by LPS, proinflammatory cytokine interferon-γ (IFN γ) and TNF-α, promoting helper T cell responses, and leads to neuronal injury by secreting the proinflammatory cytokines TNF-α and IL-1, ROS and NO by reducing the expression and release of trophic factors (Mackaness, 1964; Mosser and Edwards, 2008). M2 macrophages indicated for alternatively activated macrophages, are distinguished based on their gene expression profile. IL-10 induces an M2-deactivated, whereas IL-4 generates an M2-alternatively activated polarized phenotype. IL-4 or IL-13
12
treated macrophages hallmarked by Arginase 1 which founded in inflammatory zone and functional act such as block the release of proinflammatory cytokines, promote tissue repair, and secrete high levels of IL-4, IL-10, and IGF-1 (Song et al., 2000; Raes et al., 2002). Macrophage phenotypes and functions are dynamically regulated by the tissue microenvironment (Stout and Suttles, 2004). In spinal cord injury model, M2 macrophages loss of their phenotypes and turned into M1 macrophages (Kigerl et al., 2009).
For such reasons, microglial function cannot be determined by traditional morphological markers of microglial activation and single biochemical marker to identify a macrophage population can be problems. For example, it has been generally considered T lymphocytes act cytotoxicity, however in case of amyotrophic lateral sclerosis (Minghetti et al.) animal model, infiltrated CD4+ T lymphocytes stabilized disease progression early in disease. The results suggest that, T lymphocytes may play an endogenous neuroprotective role by modulating a beneficial M2 microglial inflammatory response (Beers et al., 2008; Chiu et al., 2008). There are descriptions of the detection markers for M1 and M2 macrophages. According to this report, M1 macrophages characterized by CD86, iNOS, CD16/32, MHC Ⅱ, IL-12, CDL15, CDL20, CXCL9, CXCL10, and CXCL11, and M2 macrophages characterized by Arginase 1, CD206, CCL18, YM1, CCL17 and so on (Mosser and Edwards, 2008; Kigerl et al., 2009).
Immune cells including CNS microglia may have both neuroprotective and cytotoxic functions depending on their different phenotype and physiologic conditions. Therefore a switch of immune cells phenotype from M1 to M2 during various inflammatory mediated disorders can be a good therapeutic strategy.
13
4. iNOS versus Arginase 1
As mentioned above iNOS and Arginase 1 are hallmark of M1 and M2 macrophages, respectively (Benoit et al., 2008). One of the enzymatic sources of NO, iNOS catalyze the oxidation of arginine to citrulline and NO (MacMicking et al., 1997). Arginin is a crucial amino acid that serves to modulate the cellular immune response during infection. It is also a common substrate for Arginase (Morris, 2010). The generation of NO from aginine is responsible for efficient immune response and cytotoxicity of host cells to kill the invading pathogens whereas the conversion of arginine to orinitihine and urea via the arginase pathway can support the tissue repair, and allergic and anti-parasitic response. The competition between iNOS and arginase for arginine can thus contribute to outcome of several parasitic bacterial infection (Munder et al., 1999).
Arginase 1 expression induced in myeloid cells by exposure to the IL-4, IL-13 (Munder et al., 1998; Munder et al., 1999), transforming growth factor-β (TGF- β; (Boutard et al., 1995), macrophage-stimulating protein(Morrison and Correll, 2002), or granulocyte colony-stimulating factor (G-CSF) (Jost et al., 2003). The transcripation factor C/EBPβ was partially required for Arginase 1 response and activation of signal transducer and activator of transcription 3 (STAT3) by the cytokines was also required for the induction of Arginase 1(Qualls et al., 2010). iNOS usually induced by IFN-γ, IL-1, TNF, IFN-α or IFN- β. These cytokines regulate various transcription factors such as nuclear factor-κB (NF-κB), activator protein 1 (AP1), IFN-regulatory factor 1 and STAT1 (Kleinert et al., 1998; Ganster et al., 2001). Arginase 1 and iNOS are competitively regulated by above cytokines.
14
arginine as a substrate for iNOS and thereby negatively regulates its enzymatic activity (Munder et al., 1999). Durante et al. have been suggested that Arginase inhibits the production of NO by several mechanisms including competing with NOS for L-arginine, affecting the translation and stability of iNOS protein, inhibiting iNOS activity via the generation of urea, and sensitizing NOS to its endogenous inhibitor, asymmetric dimethyl-L-arginine (Durante et al., 2007). And recent data have shown that Arginase inhibition by apocynin could increase arginine-dependent NO synthesis (Tossi et al., 2009).
C. Capsaicin and their receptor TRPV1 1. Capsaicin
Capsicum, the principal pungent ingredient of hot peppers, was first isolated by Thresh
in 1864 and was named capsaicin (Cash et al.). The structure of CAP, N-(4-hydroxy-3-methoxybenzyl)-8methylnon-6-enamide, was elucidated by Nelson in 1919 (Iwai et al., 1976). It is known to have tree functional regions which is homovanilic acid (A region), an amide bond (B region), and an aliphatic chain (C region). A and B regions contain dipolar groups, such as hydroxyl and carbonyl groups, which are implicated in hydrogen bonding interactions. C region has optimal chain length of 8-10 carbon atoms, is presumed to interact hydrophobically with its receptors (Walpole et al., 1993a,c; Walpole et al., 1993b).
There are numerous studies about structure-activity relationship of CAP analogues and these suggested that CAP and its analogues are powerful substance for excitation of nociceptive sensory neurons (Caterina and Julius, 2001; Calixto et al., 2005). Most CAP sensitive nociceptors are in C fibers expressing a specific receptor (Szolcsanyi, 1977). There
15
are pharmacological supporting for the existence of a vanilloid receptor (VR1) in sensory neurons which stimulated by CAP (Cholewinski et al., 1993; Koplas et al., 1997). The feature of CAP-sensitive neurons can trigger the release of neuropeptides from their peripheral terminals such as substance P (Maggi and Meli, 1988). CAP leads initially to nociceptors firing and a period of enhanced sensitivity to painful thermal and mechanical stimuli (Jancso, 1992). However they also can evoke desensitization as a period of decreased sensitivity to painful chemical, mechanical, or thermal and mild stimuli (Robbins, 2000).
The biological half-life of CAP in the rat after i.p. administration of CAP was 12.3 min. According to the results, the plasma concentration of CAP was 70.4 ng/ml at 16 min and 17.7 ng/ml at 40 min after the injection of 1 mg/kg CAP (Kawada et al., 1985).
2. Transient receptor potential vanilloid subtype 1 (TRPV1)
CAP receptor was first identified as a cDNA encoding by Caterina in 1997 using Ca2+ imaging-based expression strategy (Caterina et al., 1997). The cloned CAP receptor was designated VR1 because a vanilloid moiety constitutes an essential chemical component of both CAP and its analogues, resiniferatoxin. VR1 is selectively expressed by small- to medium-diameter neurons within dorsal root, trigeminal and nodes sensory ganglia (Caterina et al., 1997; Tominaga et al., 1998). VR1 have six transmembrane domains (TM) and a short, pore-forming hydrophobic stretch between the fifth and sixth TM domains. It is similarity with most of ion channel including cyclic nucleotide-gate channels and very similar to members of the transient receptor peotential (TRP) family of ion channel, so VR1 called TRPV1 (Tominaga and Julius, 2000).
16
In analysis the Western blotting, TRPV1 expression was wide distribution in the various brain regions including hippocampus, cortex, cerebellum, olfactory bulb, mesencephalon and hindbrain in adult rat. It has been detected in the synapses, not only neurons but also in the end feet astrocytes and pericyte (Toth et al., 2005). Recently our group proved that DA neurons and mesencephalic microglia also have TRPV1 (Kim et al., 2005). CAP has been reported to induce increased gluatamte relases from nigral slices (Hajos et al., 1988), and to produce hypokinesia in parallel to decrease in the activity of nigrostriatal DA neurons (Di Marzo et al., 2001). These results suggested that TRPV1 has a functional role in the SN.
TPRV1 have various agonist including noxious heat (~43 ℃) (Tominaga et al., 1998), low pH (pH 7.4) (Caterina et al., 1997), and lipid metabolite such as olvanil, endocannabionoid anandamide (Melck et al., 1999), and CAP (Caterina et al., 1997). The binding site of CAP on TRPV1 is N and C cytosolic tail involving the negative charge of glutamate at 761 for ligand recognition and the positive charge of arginine at 114 for ligand binding (Jung et al., 2002).
3. The neuroprotective effects of CAP and TRPV1
The main function of TRPV1 is conduction the pain pathway however recent several studies shown that TRPV1 may have neuroprotective effects. For example in ouabain-induced excitotoxicity in vivo model, TRPV1 agonist arvanil has been shown the protective effects on neurodegeneration of striatum (Veldhuis et al., 2003). Furthermore, TRPV1 completely recover of total and relative spectral power, spontaneous motor activity, memory function and hippocampal CA1 neuronal density in a model of global cerebral ischemic
17
model in mongolian gerbils. This study suggested that the protective effects of CAP may be attributable, at least in part, TRPV1 desensitization (Pegorini et al., 2005b). Another study on myocardial ischemic model, TRPV has shown endogenous protective role limiting the damage (Sexton et al., 2007). And it also protect against the onset of LPS-induced sepsis model through reducing NO and TNF-α releasing (Clark et al., 2007). Similar result has been reported that small-dose CAP reduces systemic inflammatory inflammation in sepsis model by reducing NO, TNF-α, IL-6 releasing and increasing IL-10 (Demirbilek et al., 2004).
In addition to CAP have potential role for inhibition of the inflammatory response in
vitro. It suppressed the NADH oxidase activation in various cancer cell line (Morre et al.,
1995), IκB-a degradation in peritoneal macrophages(Kim et al., 2003), and TNF- α, NOS and COX-2 induction in RAW264.7 macrophages (Chen et al., 2003; Park et al., 2004). Morever, CAP induces heme oxygenase-1, the antioxidant enzyme, expression in HepG2 cells via activation of PI3K-Nrf2 signalling (Joung et al., 2007).
According to these results, it is suggested the possibility that TRPV1 play an important role for inflammatory mediated disorder such as PD.
D. Aims of this study
The present study performed to investigation of functional effects of CAP in LPS-induced inflammatory response including the changing of phenotypes or population of infiltrated immune cells in the SN. Additionally, we investigated to whether CAP would have ability to DA neuronal protection against LPS. We also finally confirmed whether these effects of CAP were mediated TRPV1.
18
II
. MATERIALS AND METHODS
A. Materials and methods
Diagram of experimental scheme in vivo (Fig. 1).
1. Stereotaxic surgery
All experiments wereperformed in accordance with approved animal protocols and guidelinesestablished by Kyung Hee University. Female Sprague Dawley (SD) rats(230-280 g) were anesthetized by injection of chloral hydrate(400 mg/kg, i.p.) and positioned in a stereotaxic apparatusㅇ. And received a unilateral administration of PBS or LPS into the right SN (anteroposterior (AP) 5.1 mm, mediololateral (ML) 2.0 mm, dorsoventral (Medvedev et al.) 7.9 mm from bregma), according to the atlasof Paxinos and Watson (1998). All injections were made using a Hamilton syringe equipped with a 30s gauge beveled needleand attached to a syringe pump (KD Scientific, MA, USA).Infusions were made at a rate of 0.5 μl/min for LPS(5 mg in 3 μl of sterile PBS; Sigma) and for PBS as controls. After injection, the needle was left in place for an additional 10 min before slowly retracted. Animals were sacrificed and their brains harvested at indicated time pointes.
2. Pharmacological treatments
Capsaicin (Cash et al.) was obtained from Sigma-Aldrich Inc. (Saint Louis, USA), and
19
mg/kg, i.p.) 30 min before LPS intranigral injection. And CZP (1 mg/kg, i.p.) was treated 30 min before CAP. Control animals received vehicle with PBS intranigral injection. CAP and CZP were dissolved in 1 ml/kg body weight 8:1:1 v/v PBS/Tween80/absolute ethanol (Pegorini et al., 2005a). There was no difference in body weight and growth rate betweenany of the groups.
3. Tissue preparation and immunohistochemistry
Animals were anesthetizedwith chloral hydrate (400 mg/kg, i.p.) at the indicated time points after injection and transcardially perfused with salinesolution containing 0.5% sodium nitrate and heparin (10 U/ml),followed by fixation with 4% paraformaldehyde dissolved in 0.1M phosphate buffer (PB). Brains were removed from the skull,postfixed overnight at 4°C in buffered 4% paraformaldehyde,and stored at 4°C in 30% sucrose solution until they sank. Brains were frozen sectioned using a sliding microtome into40 µm coronal sections and collected in six separate series.Immunohistochemistry was performed using the avidin-biotin stainingtechnique as previously described with some modifications (Choi et al., 2005a). Free-floating serial sections were rinsed in PBS twice for 15min and then pretreated for 5 min at room temperature (RT) in PBS containing 3% H2O2. Sections were then rinsed inPBS
twice for 15min in PBS and blockedfor 30 min at RT in PBS containing 5% normal serum (Vector Laboratories, Burlingame,CA, USA), 0.2% triton X-100 (Sigma-Aldrich Inc.) and 1% BSA (Sigma-Aldrich Inc.). Sections were then rinsed inPBS containing 0.5% BSA twice for 15min. Next, the sectionswere incubated overnight with gentle shaking at RT with PBS containing 0.5% BSA and the following primary antibodies: mouse anit-neuron-specific
20
nuclear protein the NeuN (1:200; Chemicon, Temecula, CA) for general neurons, gamma-aminobutyric acid (GABA; 1:1000; Sigma) for GABAergic neurons and rabbit anti-tyrosine hydroxylase (TH; 1:2000; Pel-Freez, Brown Deer, WI) for dopaminergic neurons; mouse anti-CD11b (1:500; Serotec, Oxford, UK), which recognizes complementreceptor 3; rabbit anti-ionized calcium binding adaptor molecule 1 (Iba1; 1:1000; Wako Pure Chemical Industries, Osaka, Japan), and mouse anti-CD68 (1:100; Serotec), which recognizes specific for glycosylated lyxoxomal antigen stainfor microglia; rabbit anti-Myeloperoxicase (MPO; 1:1000; DakoCytomation, Glostrup, Denmark), which recognized for neutrophil; mouse anti-CD45 (1:1000, Millipore, Billerica, MA), which recognizes specific for monocyte; mouse anti-CD20 (1:1000; Thermo Fisher Scientific, Fremont, CA) which recognizes specific for B lymphocyte; mouse anti-RT1b (1:200; BD Pharmingen, San Diego, CA), which recognizes specific for MHC class Ⅱ; mouse anti-Nitro-tyrosine (1:50; ABCam, Cambridge, MA, USA), which recognizes NO-dependent oxidative stress; mouse anti-8-OHdG (1:300; Jaica, Shizuoka, Japan) which detects oxidative damage of DNA. Sectionswere then rinsed inPBS containing 0.5% BSA twice for 15min and incubated for 1 hour at RT in biotin-conjugated secondary antibody (1:400; KPL, Gaithersburg, MD, USA). Sections were rinsed again in PBS containing 0.5% BSA and incubated for 1 hr at RTin avidin-biotin complex kit (Vector Laboratories). After rinsing twice in PB, the signal was visualizedby incubating sections in 0.05% 3,3' diaminobenzidine in0.1M PB containing 0.003% H2O2. Sections were then rinsed
in 0.1M PB, mounted on coated slides, and viewed under a bright-field microscope (Olympus Optical, Tokyo, Japan). For Nissl staining,some of the SN tissue was mounted on coatedslides, dried for 1 hour at RT, stained with 0.5% cresyl violet(Sigma), dehydrated,
21
coverslipped, and then analyzed under abright-field microscope (Olympus Optical).
4. Double-immunofluorescence Staining
For double-immunofluorescence staining, tissue sections were processed as previously described with some modifications (Choi et al., 2005a). Briefly, free-floating sections were mountedon coated slides and dried for 30min at RT.After washing in PBS, sections were incubated in PBS containing 5% normal serum, 0.2% triton X-100 and 1% BSA for 30 min and rinsed three times with PBS containing 0.5% BSA. The sections were incubated in a combination of interleukin-1b (1:200; IL-1b; R&D systems, Minneapolis, MN), iNOS (diluted at1:200; Upstate Biotechnology, Lake Placid, NY), Arginase 1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), or CD206 (1:100; R&D system) and either anti-body against CD11b overnight at 4°C. Additionally the sections were incubated in a combination of MPO, CD68, CD45, CD20 or RT1b (1:100; R&D system) and either anti-body against iNOS or Arginase overnight at 4°C. After washing in PBS containg 0.5% BAS, the sections wereincubated simultaneously with a mixture of FITC-conjugatedchicken anti-mouse IgG (1:200, Molecular Probes, Eugene, OR) and conjugated goat anti-rabbit IgG or Cy3-conjugated donkey anti-goat IgG (1:200; Molecular Probes, Eugene, OR, USA)for 1 hour at RT. Slides were coverslipped withVectashield medium (Vector Laboratories) and viewed using anLSM 700 confocal laser scanning microscope (Carl Zeiss, Germany). To analyze the localization of different antigens indouble-stained samples, images were obtained from the samearea and merged using interactive software.
22
5. Stereological cell counts of DA neurons
The unbiased sterological estimation of the total number of the TH immunopositive (TH-ip) cells in the SN was made using the opical fractionator, as previously described in detail with some modifications (Choi et al., 2005a). This sampling technique is not affected by tissue volume change and does not requires reference volume determination (West et al., 1991). The borders of SN at all levels in the rostrocaudal axis are defiend, thereby including TH-ip cells in pars reticulate and pars lateralis in addition to pars compacta of the SN and excluding TH-ip cells in the ventral tegmental area (VTA). The sections used for counting covered the entire SN from the rostral tip of the pars compacta back to the caudal end of the pars reticulate (AP: -4.5 to -6.3 mm from bregma, accoding to the altas of Paxinos and Watson, 1998). Thisgenerally yielded 8-9sections in a series. Sampling was done using the Olympus CAST system (Compter Assisted stereological Toolbox) version 2.1.4 (Olympus Denmark A/S, Ballerup. Denmark), using an Olympus BX51 microscope, a motorized microscope stage (Prio Scientific, Focklan, MA) run by and IBM compatible computer, and a microcator (Heidenhain ND 281B, Germany), connected to the stage and feeding the computer with the distance information in the Z-axis. SN was delineated at 1.25 × objective and generate counting areas of 150 × 150 μm. A counting frame (1,612 μm 2) was placed
randomly on the first counting area and systemically moved through all counting areas until the entire delineated area was sampled. The sampling frequency was chosen so that about 130 TH-ip cells were counted in each specimen on the contralateral side. Actual counting was performed using a 100 × oil objective. Guard volume (4 μm from the top and 4-6 μm from the bottom of the section) were excluded from both surface to avoid the problem of lost