저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
A Master’s Thesis
Pentadecanoic acid as a novel anti-cancer agent in
human breast cancer stem-like MCF-7/SC cells
Ngoc Bao To
Department of Interdisciplinary Graduate Program in
Advanced Convergence Technology and Science
Graduate School
Jeju National University
Pentadecanoic
acid
aS
a
novel
anti-cancer
agent
in
human
breast
cancer
stem-like
MCF-7/SC cells
Ngoc
llao
'l'o
A
'I'hesissubmittcd in partial fulfillrnt:nt of
tht: rcrlttirc'mctrtfor
thc dcgrce of Masterof
Interdisciplinary
(lradr-ratc Programin
Advanccd(lonvcrs(lnce'l'cchrrology
and Scicr-rceI)ccc'mbcr,2020
This
thcsis has been cxamined and approved.S.
r,'
(,^'n
C
h
"
M "'*u.'
K
toL' ttwceYa
Ifl
,,:n
iae.
7
2020.12
_T)cpartrnitnt
of Intcrdisciplinarv
Graduate Program
in
Advanced Convcrgencc'l'cchnologv and
Scicnce(,raduatc
SchoolCONTENTS
ABSTRACT ... ...1
1. INTRODUCTION ... 2
2. MATERIALS AND METHODS ... 5
2.1. Cell line and cell culture ... 5
2.2. Cell viability assay ... 5
2.3. Cell migration assay ... 5
2.4. Cell invasion assay ... 6
2.5. Flow cytometric analysis of the CD44+/CD24− population ... 6
2.6. ALDEFLUOR assay ... 7
2.7. Mammosphere formation assay ... 7
2.8. Cell cycle assay ... 7
2.9. Annexin V/PI staining assay ... 8
2.10. Western blot analysis ... 8
2.11. ROS generation analysis ... 8
2.12. Statistical analysis ... 9
3. RESULTS... 10
3.1. MCF-7/SC cells exhibited higher stem cell properties than parental MCF-7 cells ... 10
3.2. Pentadecanoic acid effectively inhibits MCF-7 & MCF-7/SC cell proliferation. ... 12
3.3. Pentadecanoic acid supressed migration and invasion capacity of MCF-7/SC cells ... 15
3.4. Pentadecanoic acid suppressed the stem cell-like properties of MCF-7/SC cells ... 17
3.6. Pentadecanoic acid induce apoptosis in MCF-7/SC cells ... 22
3.7. Pentadecanoic acid enhances chemosensitivity of MCF-7/SC cells to tamoxifen ... 24
4. DISCUSSION ... 28
5. CONCLUSIONS ... 34
LIST OF FIGURES
Figure 1. MCF-7/SC cells displayed greater stemness properties than MCF-7 cells..
... 11
Figure 2. Pentadecanoic acid shows significant cytotoxic activity against MCF-7 &
MCF-7/SC cell proliferation ... 14
Figure 3. Pentadecanoic acid suppressed EMT in MCF-7/SC cells. ... 16
Figure 4. Pentadecanoic acid suppressed the stem cell-like characteristics of
MCF-7/SC cells. ... 18
Figure 5.
Pentadecanoic acid suppressed cancer the JAK2/STAT3 signaling in
MCF-7/SC cells. ... 21
Figure 6. Pentadecanoic acid induced apoptosis in MCF-7/SC cells ... 23
Figure 7. Pentadecanoic acid enhances chemosensitivity of MCF-7/SC cells to
Tamoxifen. ... 26
ABSTRACT:
Compelling evidence from biological and nutritional studies recently has further reinforced the importance of odd-chain fatty acids (OCFAs) in health benefits. Very little is known about the biological mechanism of OCFAs as anti-cancer agents. Pentadecanoic acid (C15:0) is one of the common OCFAs. In this study, we found that pentadecanoic acid could suppress the stemness of the breast cancer stem-like cells MCF-7/SC. MCF-7/SC were established from MCF-7 breast carcinoma cells, which exhibit high stem cell properties. Our results demonstrated that pentadecanoic acid has specific cytotoxicity on MCF-7/SC compare to its parental cells. In addition, pentadecanoic acid dramatically suppressed the stemness by targeting CSC markers, mammosphere formation, and migration as well as invasion ability of MCF-7/SC cells. Moreover, we found that pentadecanoic acid can suppress IL-6-induced JAK2/STAT3 activation, induce sub-G1 accumulation and promote caspase-dependent apoptosis in breast MCF-7/SC cells. In addition, pentadecanoic acid enhanced the chemosensitivity of MCF-7/SC cells to tamoxifen, a commonly used chemotherapy in breast cancer treatments. These findings indicate that pentadecanoic acid can serve as a novel JAK2/STAT3 signaling inhibitor in breast cancer cells and suggest the beneficial effects of pentadecanoic acid-rich food intake during breast cancer treatments.
1. INTRODUCTION
Breast carcinoma is one of the common causes of death worldwide among women [1]. Advanced breast cancer screening methods and therapeutic strategies such as surgery, chemotherapy, and radiation therapy has enhanced the chances of survival of patients remarkably by contributing to a vital eliminate of the primary tumor [1]. Even if breast cancer is diagnosed and treated at an earlier stage, some continuing cells remain and developing some time leading to local recurrence which seriously endangers the status of life and even reduces survival [2]. In recent years, there has been valid evidence implies that the underlying existence of a small subpopulation could be found during any stage of breast cancer progression that is capable for causing the tumor progression, as well as therapeutic resistance, termed breast cancer stem cells (BCSCs) [3]. So far, severral BCSC markers have been identified such as CD44, CD24, CD133, EpCAM, CD166, CD47, P-glycoprotein (P-gp/ABCB1), multidrug resistance-associated protein 1 (MRP1/ABCC1) and ABCG2 [4]. Moreover, aldehyde dehydrogenases (ALDH) activity and epithelial-to-mesenchymal transition (EMT) processes have been utilized as selective markers in CSCs that have been correlated with a poor prognosis of breast cancer [5]. The expression of ATP-binding cassette (ABC) efflux transporters in breast cancer cells including P-gp/ABCB1 and MRP1/ABCC1 restrics the exposure to anti-cancer drugs and reduce significantly the success of cancer therapy [6]. Therefore, new cancer therapies that can repress cancer stem cell populations are important and essential.
Various intracellular signaling pathways play an essential role in the CSCs proliferation and progression such as Hedgehog, Wnt, NF-κB, PI3K/AKT/mTOR, TGF/SMAD, Notch, PPAR, JAK2/STAT3, TGF/SMAD, PPAR [7]. Among these, JAK2/STAT3 participated in
many essential processes of cancer cells. JAK2/STAT3 signaling pathway involved in the EMT process has been reported as one of the signaling pathways involved in CSCs generation [8]. In breast cancer, STAT3 approximates is overexpressed in 50-60% cases [9]. Previous studies demonstrated that activated STAT3 plays a major role in breast cancer tumorigenesis. Recently, a number of drugs have been identifed the JAK2/STAT3 signaling pathway inhibitors.
Saturated fatty acids play an essential role as antecedents of many signaling molecules in the cell [10]. The majority of saturated fatty acids are found in both plant and animal tissues [11]. Saturated fatty acids were classified not only by saturation or unsaturation but also according to their number of carbon atoms including OCFAs and even chain fatty acids [11]. The human plasma contains about 99% of even chain fatty acids. Among these OCFAs, heptadecanoic acid (C17:0) and pentadecanoic acid (C15:0) are the most popular ones [12]. Accumulating evidence indicated that pentadecanoic acid and heptadecanoic acid can be serve as markers for dairy consumption evaluation, coronary heart disease risk as well as type II diabetes mellitus risk [13]. Interestingly, recent investigations have reported that OCFAs are also plays as an cancer agent [14, 15]. Xu et al. (2019) demonstrated anti-cancer effects of heptadecanoic acid (C17:0) in non-small cell lung carcinomas (NSCLC) by [16]. However, possible anti-cancer effects of pentadecanoic acid in breast cancer therapy, remains unexplored.
In the present investigation, we provided for the first time that the anti-cancer effects of pentadecanoic acid in human BCSCs. Pentadecanoic acid was found to suppress the stem cell characteristics of MCF-7/SC cells. Besides, the downregulation of JAK2/STAT3 signaling and induction apoptosis upon pentadecanoic acid exposure was also evident. Our findings
may provide a better understanding of OCFAs through the anti-cancer activity of pentadecanoic acid, a potential agent of preventing disease in human systems.
2. MATERIALS AND METHODS
2.1. Cell lines and cell culture
The Human BCSCs MCF-7/SC were established from MCF-7 cells by sorting CD44+/CD24− population in the Stem cell Institute, Vietnam. MCF-10A cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). 7, MC-7/SC, MCF-10A cells were maintained in DMEM, RPMI-1640, FBMTM Fibroblast Growth Basal Medium
(Lonza Group Ltd, Switzerland), respectively. All media were mixed with fetal bovine serum (10% FBS) and antibiotics containing 100 U/mL penicillin and 100 µ g/mL streptomycin (1%).
2.2. Cell viability assay
In this investigation, 96-well plates were used for culturing cells. Initially, 200 µ L of MCF-7/SC (104 cells/mL,) were cultured for 24 h following by treating with different
concentrations of C15:0, C17:0, C18:1 or C18:2. All the fatty acids were obtained from the Sigma, St. Louis, MO, USA and filtered-EtOH was used as solvent. MTT assay was then conducted as previously described [17]. The GraphPad Software (GraphPad Prism 7.00) was used to determine the half maximum inhibitory concentration (IC50) of pentadecanoic acid
in each breast cancer cell line.
2.3. Cell migration assay
Cells (1.5 × 105 cells/well) were cultured in 6‑well plates until the cells reach confluency
were made. Cells were then washed with PBS until detached cells were removed. In the next step, MCF-7/SC were exposed to pentadecanoic acids for 48h. After incubation, the width of the wound areas were observed and measured through an inverted phase-contrast microscope at 4× magnification.
2.4. Cell invassion assay
The invasive capacity of cells was evaluated using 24-well plates. This is a Transwell system purchased from Corning, Cambridge, MA, USA. The upper chambers were precoated with Matrigel diluted in cold serum-free media before seeding. MCF-7/SC were suspended in serum-free medium at a density of 6 × 105 cells/mL). 200 µ L of cells were added to each Transwell comprising with or without 50 µ M pentadecanoic acid. Then, the lower chamber was filled with 750 µ L of RPMI-1640 (10% FBS). After 48 h of treatment, cells were washed with PBS twice. 4% paraformaldehyde were used for fixing MCF-7/SC, and following by methanol. In the last step, invading cells were stained with crystal violet (2%) and captured by using a phase-contrast microscope.
2.5. Flow Cytometric Analysis of the CD44+/CD24− Population
To investigate the effect of pentadecanoic acid on the cell surface expression of CD24 and CD44, the flow cytometry (BD Biosciences, San Diego, CA, USA) was used. MCF-7/SC were seeded into 60 mm dishes at a density of 2 × 104 cells/mL for 24 h. Different
concentrations of pentadecanoic acid then was added to PRMI 1640 (10% FBS, 1% antibiotics). After 48 h of treatment, cells were harvested and washed twice with PBS. Then, MCF-7/SC cells were re-suspended in immunofluorescence staining buffer (100 μL) and incubated at 4 °C (10 - 15 minutes). This buffer contained PE-conjugated anti-human CD24
antibody and FITC-conjugated anti-human CD44 antibody. Both the antibodies were obtained from BD Pharmingen, San Diego, CA, USA. After harvesting, the stained cells were washed with 0.5 mL PBS and performed the analysis.
2.6. Aldefluor Assay
ALDEFLUOR Kit was obtained from Stemcell Technologies, Vancouver, BC, Canada. ALDH enzyme activity was analyzed using ALDEFLUOR Kit following the manufacturer’s instruction. Diethylaminobenzaldehyde (DEAB) was used as the negative control. MCF-7/SC were seeded at density 2 × 104 cells/dishes (60 mm dish). MCF-7/SC cells were treated
with non-lethal concentration of pentadecanoic acid for 48 h. Then, the analysis was carried out using the FACSCalibur flow cytometer.
2.7. Mammosphere Formation Assay
The ultralow-attachment dishes were used to cultured MCF-7/SC (2 × 104 cells/mL).
MammoCult Human Medium (Stemcell Technologies) was used as the media for mammosphere formation assay. After 10 days, mammospheres were observed under a phasse-contrast microscope. Mammospheres that have a diameter larger than 60 μm were counted.
2.8. Cell cycle assay
MCF-7/SC cells were cultured at density 3 × 104 cells/dish (60 mm dish) and then treated
with or without pentadecanoic acid. Then, MCF-7/SC cells were harvested and washed with PBS twice. Following centrifugation, 70% EtOH was used to fix MCF-7/SC. Before performing the analysis, fixed cells were stained with 500 µ l solution consist of 25 ng/mL RNase A, 30 μg/mL PI, and 2 mM EDTA-PBS (RNase A: PI: EDTA-PBS = 10 µ l: 1 µ l: 3 mL)
and incubated at 37 °C at least 10 minutes in the dark. The stained cells were analyzed the cell cycle by using the FACSCalibur flow cytometer.
2.9. Annexin V/PI staining assay
The annexin V-FITC Apoptosis Detection Kit I was used to conduct the annexin V-FITC assay according to manufacturer’s protocol. Initially, MCF-7/SC cells were cultured at density 3 × 104 cells/dish (60 mm dish). Then, different concentrations of pentadecanoic acid
was supplemented in RPMI 1640 (10% FBS, 1% antibiotics) for 48h. Then, cells were trypsinized, and washed with PBS twice. Propidium iodide (PI) and annexin V-FITC were diluted 50 times and 20 times in 1x binding, respectively. PI and annexin – FITC conjugate then were added in the cells, mixed gently. Stained cells were incubated at room temperature in the dark (15 minutes). The analysis was conducted using the FACSCalibur flow cytometer.
2.10. Western blot analysis
After 48 h treatment with pentadecanoic acid, MCF-7/SC were lysed with RIPA lysis buffer containing protease inhibitors and PMSF (Beyotime, China) to extract total protein. BCA assay was used to quantify protein.
Western blot experiments were performed as
previously mentioned in our recent publication [17].
The primary antibodies were obtainedfrom Cell Signaling Technology, Inc, United States. The secondary antibodies, the anti-rabbit and the anti-mouse immunoglobulin G (IgG) were purchased from Vector Laboratories, Burlingame, CA, USA). The BS ECL Plus Kit was purchased from Biosesang, Seongnam, South Korea to detect bands.
ROS generation assay was conducted using the FACSCalibur flow cytometer. Briefly, cells were seeded at a density of 3 × 104 in 60 mm dishes. Following 48 h of incubation,
harvesting and staining, the cells were treated with 2′,7′-dichlorofluorescein diacetate (H2DCFDA) for 15 minutes. After, PBS was used to wash stained cells, and then performed the ROS generation analysis.
2.12. Analysis
Group comparisons were performed using the Student's t-test and one-way analysis of variance with GraphPad Prism 7.0 (La Jolla, CA, USA) software. P-values < 0.05 were considered statistically significant.
3. RESULTS
3.1. MCF-7/SC cells exhibited greater stem cell properties than the parental MCF-7 cells
To examine one of the properties of stem cells, we used the flow cytometry technique to compare the CD44+/CD24- population in MCF-7/SC and parental MCF-7 cells. Results displayed that the cell surface markers (CD44+/CD24-) were enriched in MCF-7/SC cells compared to their parental cells (Figure 1a). We next investigated the reactive oxygen species (ROS) level and found a lower ROS level in MCF-7/SC (Figure 1b). Other evidence also indicates that there has been an increase in mammosphere formation capacity in MCF-7/SC cells (Figure 1c). Moreover, Western blot experiments were conducted to detect the changes in the protein expression of stem cell markers, including MDR1, MRP1 and CD44 which were dramatically higher whereas CD24 was lower in MCF-7/SC cells (Figure 1d). Similarly, a higher cell migration capacity in MCF-7/SC cells compared to MCF-7 cells was also illustrated in Figure 1e. Altogether, these results indicated that MCF-7/SC cells displayed greater stem cell properties than the parental MCF-7 cells.
Figure 1. MCF-7/SC cells displayed greater stemness properties than MCF-7 cells. (a) Comparison of CD44+/CD24- population by using FACs. (b) ROS levels were measured after
staining with H2DCFDA by flow cytometry analysis (100× magnification). (c) Mammosphere formation capacity by maintaining cells in complete MammoCult Human Medium for 10 days. (d) The protein level of cancer stem markers were investigated by western blot analysis. GAPDH was used as the loading control (e) Comparison of migration capacity in MCF-7/SC and MCF-7 by the Wound healing assay; results are presented as mean ± SD. (* p < 0.05).
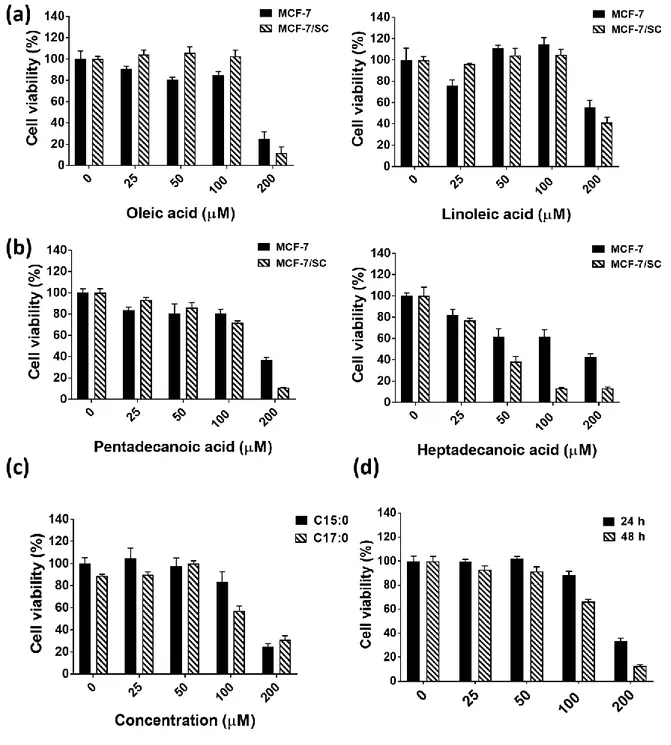
3.2. Pentadecanoic acid effectively inhibits MCF-7 & MCF-7/SC cell proliferation.
Various fatty acids may exert growth inhibitory effects depending on the cancer cell type. It has been reported that the saturated acid - oleic acid (C18:1) effectively inhibited the proliferation of breast [18], Tongue Squamous [19] and esophageal [20] cancer cells. Another unsaturated fatty acid, linoleic acid (C18:2) has been reported to exert cytotoxicity in colorectal cancer cells [21]. Therefore, screening of these fatty acids may provide the potential candidates for cancer therapy.
To investigate the effect of saturated fatty acids including pentadecanoic acid (C15:0), heptadecanoic acid (C17:0), and unsaturated fatty acid including oleic acid (C18:1), and linoleic acid (C18:2) in breast cancer cells, the MTT assay was conducted. As illustrated in Figure 2a, oleic acid and linoleic acid (unsaturated fatty acids) exhibited less cytotoxic in both MCF-7 and MCF-7/SC cells compared to pentadecanoic acid and heptadecanoic acid (saturated fatty acid). The effect of pentadecanoic acid in MCF-7 and MCF-7/SC cells was also compared in Figure 2b. As IC50 values of 41.94±4.06 µ M, heptadecanoic acid displayed
119±5.21 µ M). Heptadecanoic acid exerted the cytotoxic effects whereas pentadecanoic acid did not affect the proliferation of MCF-10A (non-tumorigenic epithelial cells) (Figure 2c), pentadecanoic acid may be an OCFA reliable for further experiments. Pentadecanoic acid treatment resulted in a reduction of MCF-7/SC cell viability and its cytotoxic were dose-dependent, with IC50 values of 155.5±9.55 µ M for 24 h post-incubation and 119±5.21 µ M for
48 h post-incubation (Figure 2d). Altogether, these results suggest that pentadecanoic acid is a suitable candidate for breast cancer treatment.
Figure 2. Pentadecanoic acid shows significant cytotoxic activity against 7 & MCF-7/SC cell proliferation. (a) The effect of unsaturated fatty acids: oleic acid (C18:1) and
linoleic acid (C18:2) on breast cancer cells for 48 h. (b) The effect of saturated fatty acids: pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) for 48 h. (c) The cytotoxic effect of saturated fatty acid on MCF-10A on breast cancer cells after 48 h. (e) The effect of
pentadecanoic acid treatment on MCF-7/SC cells for 24 h and 48 h. Results are shown as mean ± SD. (* p < 0.05).
3.3. Pentadecanoic acid supressed the migration and invasion capacity of MCF-7/SC cells
We evaluated whether pentadecanoic acid could inhibit the cell motility of MCF-7/SC cells by using wound healing, invasion assays, and Western blot. As presented in Figure 3a and 3b, pentadecanoic acid treatment at non-lethal concentration significantly supressed the migration and invasion capacity of MCF-7/SC were compared with the untreated group (Figure 3a, 3b). Previous studies have indicated that EMT has an important role in enhancing cancer cell motility. The anti-migration effects and anti-invasion effects of pentadecanoic acid were determined, we further examined the underlying mechanism of its action. Matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9) are the members of MMPs - proteolytic enzymes of the extracellular matrix (ECM), closely related to EMT process of various types of cancer cells. Following this, the protein expressions of MMP2 and MMP9, as well as other EMT markers including Snail and Slug were detected to evaluate the effect of pentadecanoic acid on the motility capacity. Pentadecanoic acid remarkably decreased the expression of EMT-associated protein such as snail, slug, MMP2, and MMP9 on MCF-7/SC. All of these data indicated that pentadecanoic acid treatment could supress cell motility of MCF-7/SC cells.
Figure 3. Pentadecanoic acid suppressed EMT in MCF-7/SC cells. (a) The wound healing assay was perfomed to evaluate the effect of pentadecanoic acid on cell migration. (b) Effect of pentadecanoic acid on cell invassion was evaluated by the Trans-well invassion assay. (c) Western blot analysis of EMT related-markers was conducted following 48 h of incubation with pentadecanoic acid. GAPDH was used as a loading control; Results are presented as mean ± SD. (* p < 0.05).
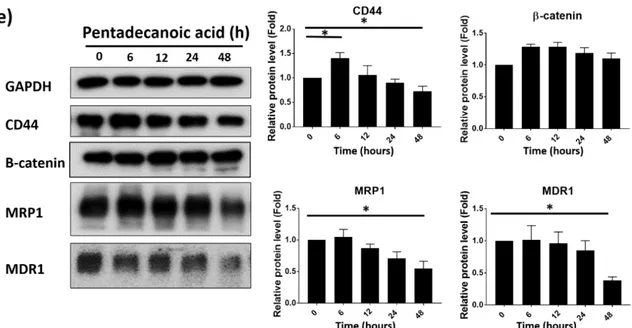
3.4. Pentadecanoic acid suppressed the stem cell-like properties of MCF-7/SC cells
As we found that pentadecanoic acid has more cytotoxic activity on MCF-7/SC cells than MCF-7 cells, we examined whether this OCFA could suppress the CSC population of MCF-7/SC cells. The characteristics of BCSCs were identified based on CD44+ /CD24-- expression, the enzymatic activity of ALDH and possess a capability to form
mammospheres in non-adherent cultures. As illustrated in Figure 4a, the mammospheres formation capacity of MCF-7/SC cells was significantly diminished by pentadecanoic acid treatment. Furthermore, pentadecanoic acid treatment dramatically decreased the percentage of CD44+/CD24- cell population in a dose-dependent manner (Figure 4b). Pentadecanoic acid treatment resulted in a reduction of ALDH activity was also observed in Figure 4c. To confirm this, we evaluated the effect of pentadecanoic acid on expression CSC markers by Western blot analysis. The results in Figure 4e and 4f, pentadecanoic acid caused decrease in expression levels of CSC markers such as MRP1, MDR1, CD44 and β-catenin, in a dose-dependent manner (Figure 4e) and in a time-dependent manner (Figure 4f). All of these data demonstrated that definite characteristics of CSCs are effectively suppressed by pentadecanoic acid treatment.
Figure 4. Pentadecanoic acid suppressed the stem cell-like properties of MCF-7/SC cells.
(a) Effects of pentadecanoic acid on mammosphere formation of MCF-7/SC (100×
magnification). (b,b’) FACs analysis showing reduction of CD24-/ CD44+ population on
MCF-7/SC cells. (c) ALDEFLUOR assay kit of MCF-7/SC after exposure to pentadecanoic
acid for 48
h
. DEAB used as a negative control. (d) Western blot analysis for CSC markersafter pentadecanoic acid (0, 50, 100, 150, 200 μM) treatment for 48 h. (e) Western blot analysis for CSC markers after pentadecanoic 100 μMtreatment for 6, 12, 24, 48 h. GAPDH was used
as a loading control ; Results are shown as mean ± SD. (* p < 0.05).
3.5. Pentadecanoic acid suppress cancer the JAK2/STAT3 signaling in MCF-7/SC cells.
Previous study reported that STAT3 can regulate several target oncogenes in breast carcinoma cells [22]. Previous studies reported that the activation of JAK2/STAT3 signaling play an essential role in breast CSCs development and progression included survival, apoptosis, metastasis, and chemoresistance [23]. Therefore, based on the effects of pentadecanoic acid on stem cell-like properties in MCF-7/SC, we examined whether treatment with pentadecanoic acid could inhibit JAK2/STAT3 signaling in MCF-7/SC. The
Western blot results indicated that pentadecanoic acid expose decreased the expression of JAK2, pJAK2, STAT3, pSTAT3 proteins in a dose-dependent manner (Figure 5a). The effect of pentadecanoic acid was supported by the diminished expression of these proteins in a time-dependent manner as illustrated in Figure 5b. Although pentadecanoic acid treatment could reduce both of total form and phosphorylated form of these protein, a dramatic reduction of the phosphorylated form compared to the total form were observed in Figure 5a and 5b. These data demonstrated that pentadecanoic acid attenuated the JAK2/STAT3 signaling pathway.
Previous studies reported that many upstream proteins that can activate JAK2/STAT3 signaling including Interleukin-6 (IL-6) [24-26], and the repression of IL-6/JAK2/STAT3 signaling activation leading to reduce migration, invasion as well as tumor aggressiveness of breast cancer[27]. To further understand the biological mechanism of pentadecanoic acid, we examined whether pentadecanoic acid treatment can prevent the function of IL-6 from activation JAK2/STAT3 signaling in MCF-7/SC cells. Pre-treat MCF-7/SC cells with Pentadecanoic acid at 150 M for 48 h. Before extracting protein from cells, stimulate MCF-7/SC with 20 ng/mL IL-6 for 15 mins. Our obtained result in Figure 5c showed an increase of pJAK2 and pSTAT3 protein levels caused by IL-6 stimulation in MCF-7/SC cells. Nevertheless, pentadecanoic acid can dramatically inhibit the induction of phosphorylation of the JAK2/STAT3 level by IL-6. These data provided evidence for the novel role of pentadecanoic acid as an inhibitor of the IL-6/JAK2/STAT3 signaling pathway.
Figure 5. Pentadecanoic acid inhibited the JAK2/STAT3 signaling in MCF-7/SC cells. (a)
Effects of different concentration pentadecanoic acid treatment for 48h on JAK2/STAT3 signaling in MCF-7/SC. (b) Effects of 100 µ M pentadecanoic acid treatment for 6, 12, 24, 48h on JAK2/STAT3 signaling in MCF-7/SC. (c) Effects of 150 µ M pentadecanoic acid treatment for 48h on preventing IL-6-induced JAK2/STAT3 signaling after 48h from treatment of
Pentadecanoic acid. GAPDH was used as a loading control; Results are presented as mean
± SD. (* p < 0.05).
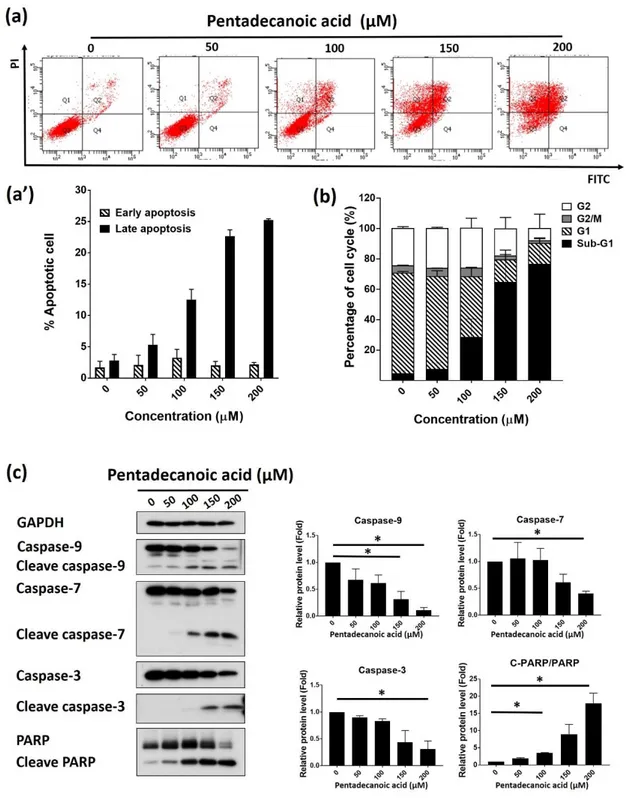
3.6. Pentadecanoic acid induce apoptosis in MCF-7/SC cells
Evasion of apoptosis is a basic feature of human cancer [28]. Screening natural compounds can kill cancer cells by inducing apoptosis is the promising strategy for cancer
therapeutic [29-31]. Multiple studies have determined that the STAT3 signaling represses
apoptosis in cancer cells, includes breast cancer cells [32, 33]. Based on this, we evaluated the effect of pentadecanoic acid in promoting cell death by inducing apoptosis in MCF-7/SC. We first analyzed MCF-7/SC cells after exposing with pentadecanoic acid at different concentration for 48 h, then AnnexinV/PI staining was performed to detect apoptosis population. The obtained results demonstrated the significance of late apoptosis by pentadecanoic acid and some early apoptosis at 48 h (Figure 6a). The connection between the induction of apoptosis and cell cycle arrest was reported in previous studies [34], we then investigated whether pentadecanoic could induce cell cycle arrest. As shown in Figure 6b, pentadecanoic acid treatment resulted in induction of sub-G1 accumulation compared with non-treated control cells. These data were further supported by Western blot results, in which increased expression of cleave form of apoptosis markers such as caspase-3,
caspase-7, caspase-8, caspase-9, PAPR. In addition, a decreased expression in total form of these proteins were also observed (Figure 6c).
Figure 6. Pentadecanoic acid promoted apoptosis in MCF-7/SC cells. (a) Results obtained after cell cycle analysis of MCF-7/SC cells treated with pentadecanoic acid at 0, 50, 100, 150
and 200 µ M for 48 h. (b) Results obtained after Annexin V/PI staining of MCF-7/SC cells treated with pentadecanoic acid at 0, 50, 100, 150 and 200 µ M for 48 h. (c) Protein levels of apoptosis markers were examined by western blot analysis following pentadecanoic acid (0, 50, 100, 150, 200 μM) expose for 48 h. GAPDH was used as a loading control; Results are shown as mean ± SD. (* p < 0.05).
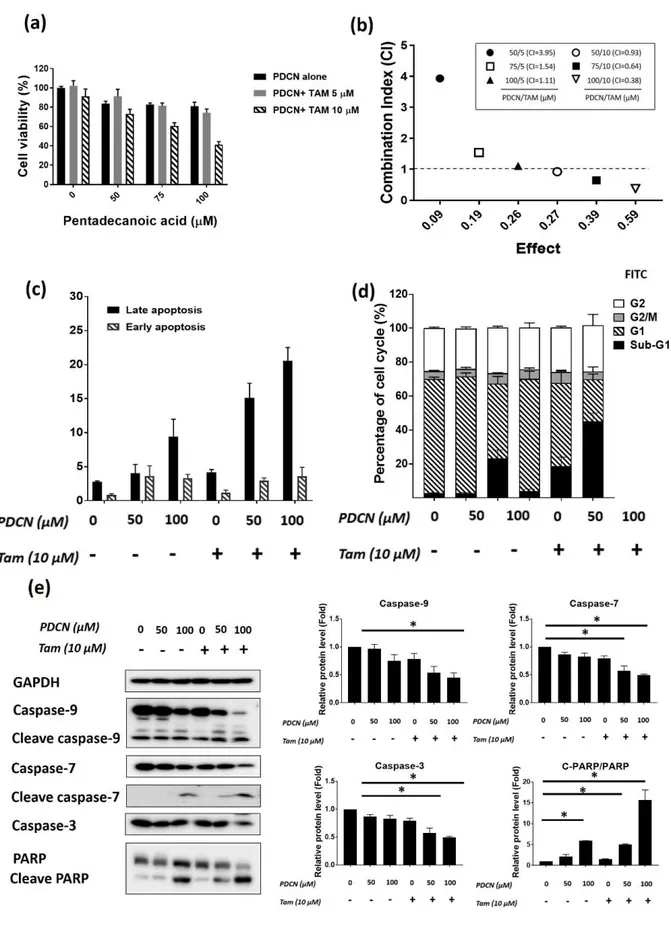
3.7. Pentadecanoic acid enhances chemosensitivity of MCF-7/SC cells to Tamoxifen
To evaluate whether pentadecanoic acid enhances cytotoxic efficacy of tamoxifen (TAM)
in-vitro, MCF-7/SC cells were co-treated with pentadecanoic acid (50 and 100 μM) and TAM
(10 μM) for 48 h and cell viability was then examined by the MTT assay. A reduction in the cell viability of MCF-7/SC was observed following treating with pentadecanoic acid and TAM compared to the TAM treatment alone (Figure 7a). To explore whether pentadecanoic acid and TAM had synergistic cytotoxic effects in MCF-7/SC cells, we calculated the combination index (CI) values. Combined treatment containing 100 μM of pentadecanoic acid and 10 μM of TAM after 48 h expose displayed highest synergistic inhibitory effects with a CI value of 0.38 (Figure 7b). As illustrated in Figure 7c, Annexin V/PI staining showed that combined treatment of pentadecanoic acid (50 and 100 μM) and TAM (10 μM) can significantly promote apoptosis in MCF-7/SC cells compared to the control, TAM treatment only or pentadecanoic acid treatment alone. Cell cycle analysis resulted in an increase in the sub-G1 accumulation of MCF-7/SC cells after co-treatment with pentadecanoic acid and TAM (Figure 7d). Highest sub-G1 population (50.05±3.78%) of MCF-7/SC cells was observed at the combined treatment containing 100 μM of pentadecanoic acid and 10 μM of TAM
(Figure 7d). Furthermore, pentadecanoic acid and TAM combined treatment were further supported by western blot analysis. As illustrated in Figure 7e, the combine treatment of pentadecanoic acid and TAM resulted in an increased cleavage form of 7, caspase-9 and PARP, whereas resulted in a reduction of the total form of these apoptosis markers. Based on these observations, we conclude that pentadecanoic acid can enhance the in-vitro cytotoxic efficacy of TAM and promote TAM induced apoptosis in MCF-7/SC cells.
Figure 7. Pentadecanoic acid enhances chemosensitivity of MCF-7/SC cells to Tamoxifen. (a) Results obtained after MTT assay performance on MCF-7/SC cells co-treated with
pentadecanoic acid at 25, 50, 75, and 100 µ M and tamoxifen (TAM) at 10 µ M for 48 h. (b) Combination Index (CI) calculated for various concentrations of pentadecanoic acid and TAM treatment in MCF-7/SC cells. (c) Results obtained after cell cycle analysis of MCF-7/SC cells co-treated with pentadecanoic acid (50, 100 µ M) and TAM 10 µ M for 48 h. (e) Results obtained after Annexin V/PI staining of MCF-7/SC cells co-treated with pentadecanoic acid (50, 100 µ M) and TAM 10 µ M for 48 h. (e) Protein levels of apoptosis markers were examined by western blot analysis following pentadecanoic acid (50, 100 μM) plus TAM 10 μM exposure for 48 h. GAPDH was used as a loading control Results are shown as mean ± SD. (* p < 0.05).
4. DISCUSSION
The CSCs play a key role in maintaining the tumor heterogeneity, driving cancer growth and drug resistance [35]. Therefore, the exploration of novel drug that can target cancer stem cells is a potential therapeutic to overcome therapy resistance. In recent years, fatty acids have become potential anti-cancer drug in cancer biology. However, still debate about particular types of fatty acids and their effects on cancer treatment. For instance, numerous types of fatty acids are not similar concerning their effects on breast cancer cell growth and death: the monounsaturated fatty acid oleate (C18:1) promotes the survival, whereas the anti-cancer function of saturated fatty acid palmitate (C16:0) was demonstrated by inducing apoptosis [36]. Saturated fatty acid, special is even chain fatty acid induce cell death have also reported in vitro and in vivo [15, 37-41]. Moreover, recent studies confirmed that effects of fatty acids against several types of cancer cells, e.g. lung cancer [16]. This compelling evidence indicated that OCFAs may exhibit anti-cancer effects on cancer cells. Nevertheless, little information exists about the anti-tumor mechanism by OCFA influence breast cancer cell survival and metastasis, especially BCSCs. Our study showed that pentadecanoic acid, one of the most common compounds belong to OCFA, has a strong cytotoxic activity against human breast cancer stem cell line MCF-7/SC (Figure 2b,e). In this work, we examined the cytotoxic activity of pentadecanoic acid in non- breast cancer stem cell line (luminal-like) breast cancer cell line (MCF-7), breast cancer stem cell line (MCF-7/SC) and non-tumorigenic breast epithelial cell line (MCF-10A). Interestingly, pentadecanoic acid displayed selective cytotoxicity against BCSCs (MCF-7/SC) compared with luminal breast cancer MCF-7 (Figure 2d) whereas the highest concentration exerted non-cytotoxic effect on
non-tumorigenic breast epithelial cell line (MCF-10A) (Figure2c), indicating pentadecanoic may play as a novel anti-cancer reagent for BCSC.
In breast cancer patients, levels of cancer stem cells have positively correlated with the risk of poor prognosis, which may enhance the chemoresistance and metastasis in patients with highly malignant [42]. In this study, we demonstrated that the breast cancer stem cell line MCF-7/SC, which isolated from MCF-7 breast cancer cells showed more prominent stem cell properties than their parent cells by enhancing the CD44+/CD24- populations,
accumulating lower ROS levels, increasing mammosphere formation as well as migration capacity (Figure 1a-e). Up-to-date evidence suggests that ALDH activity was accepted as a hallmark of cancer CSCs [4]. Moreover, MCF-7/SC cells displayed greater-level proteins including CD44, MDR1, and MRP1 than MCF-7 (Figure 1d). These proteins are CSC markers that frequently used [4]. Based on the selective cytotoxic effect on MCF-7/SC (Figure 2d), we hypothesis that this OCFA could eliminate the CSC population. To evaluate the effect of pentadeacanoic acid, we first examined the activity of pentadecanoic acid against BCSCs properties. Ours obtain results provided compelling evidence as to dramatically reduce the CD44+/CD24- population, the significant suppression of mammosphere evolution as well as
ALDH activity (Figure 4a-c). As one of the most typical CSC markers and critical regulators of cancer stemness, CD44 is responsible for self-renewal, cell invasion and migration [43]. Therefore, CD44 is allowed as a marker for isolating or enriching CSCs by using separate or in combination with other cell surface markers [43]. As anticipated, upon pentadecanoic acid treatment, the CSC markers including CD44, β-catenin, MDR1, and MRP1 decrease significantly (Figure 4d-e). This is the first report that describes the effects of OCFA against BCSCs in vitro.
The EMT program is a cellular event that cells can move to another site. This process based on lose apical-basal polarity and cell-cell adhesion of epithelial cells [44]. EMT process is essential for tumor development by intensifying migratory, invasive properties, and becomes mesenchymal stem cells [45, 46]. The direct correlation between EMT progression and the development of CSCs have been demonstrated in the previous study, implying that the EMT program plays a deciding role in the generation and maintenance of CSCs or CSC-like cells [47, 48]. Up to now, many cellular and molecular related to EMT processes have been identified, and the most critical attention is the changes associated with a class of extracellular proteases, the MMPs [49]. Among the members, MMP2 and MMP9 are two well-known members of the MMP gene family [50, 51]. MMP2, also known as gelatinase A, promoted the malignant phenotype of cancer cells through causing the breakdown of the basement membrane and enhancing the local and distant invasion of tumor cells [52]. Besides MMP2, MMP9 (gelatinase B) driven angiogenesis by interrupting in the regulation of growth plate and recruitment of endothelial stem cells [53]. Previous studies also accepted both Snail and Slug are the zinc-finger regulatory transcription factors required in the EMT process of cancer cells, which is related to the aggressive clinical phenotype in breast cancer [54]. Our results found that the migration and invasion ability of MCF-7/SC significantly repressed upon pentadecanoic acid treatment (Figure 4a-b). Correlating with this, the expressions of MMP2, MMP9, Snail, and Slug also remarkably decreased compared with no treatment (Figure 4c), suggesting that pentadecanoic acid could suppress cell motility capacity through the suppression of EMT-related protein expression in MCF-7/SC cells.
A number of clinical evidence showed that the signal transducer and activator of transcription 3 (STAT3) are constitutively activated in almost cancer, covering more than 40% of all breast cancer [55]. As an essential gene in generation and survival of the cancer cells,
STAT3 participates in cell proliferation, apoptosis, metastasis, and other cellular happenings including EMT in breast cancer [56-60]. Succinctly, when growth factors or cytokines bind to the related receptors on the cell surface, it leads to activation of receptor-associated tyrosine kinases [61]. The most notable is the Janus kinase, JAK family of kinases, this process leading to the recruitment and constitutive activation of STAT3 on the Tyr-705 residue [61]. The dimerization and nucleus translocation processes take place immediately after STAT3 was activated. Activated STAT3 acts as a regulator factor that can regulate the transcription of the target gene such as Bcl-2 families, c-Myc, Survivin, MMP2, MMP9 via binding to the interferon-gamma activated sequence (GAS) of promoters [59, 62-64]. A recent study indicated that U-STAT3 involved in the regulation of gene expression through bind to GAS sequences as a dimer or monomer [65, 66]. In the present investigation, upon treatment with pentadecanoic acid at both time and dose-dependent manner significantly inhibits JAK2/STAT3 signaling as shown in Figure 5a-b, provided the new biological function of pentadecanoic acid in BCSCs as a potent inhibitor of the JAK2/STAT3 pathway. IL-6, a pro-inflammatory cytokine accepted as one of the most well-known upstream activators of the JAK2/STAT3 pathway [67]. As expected, our results showed in Figure 5c indicated that exposure to pentadecanoic acid resulted in a suppression IL-6-induces the JAK2/STAT3 signaling pathway. Taken together, these results provided compelling evidence for the first time that pentadecanoic acid can suppress cancer stem cell properties, decrease migration, and invasion capacity via inhibiting JAK2/STAT3 signaling.
As aforementioned, STAT3 regulates the transcription of a wide range of genes involved in apoptosis by binding to the specific promoter region both pro- and anti-apoptotic families [68, 69]. Hence, we hypothesis that the cell death mechanism behind processes was apoptosis. Play a critical role in regulating apoptotic processes, caspase, a
family of cysteine proteases, are accepted as the key mediators can cleave main cellular proteins [70]. There are two classes of caspases: the initiator caspases and the effector caspases [70]. The initiator caspases included caspase-3, csaspase-8, csaspase-9 and csaspase-10 whereas the effector caspases included csaspase-3, csaspase-6 and csaspase-7 [71]. Important, activation of caspase-3 is the central phenomenon that responsible for most of the cleavage events during the apoptosis process [72]. (ADP-ribose) polymerase-1 (PARP-1) is popular cellular substrates of caspases. One of the various events of apoptosis that have been accepted is the cleavage of PARP-1 by caspases [73]. Consistent with the reported finding of the critical role of caspases and PARP-1 during the apoptosis, our results showed a heightened expression of cleave caspase-3, -7, -8, -9 in the pathway upon pentadecanoic acid treatment (Figure 6a-c), which indicated the pentadecanoic acid treatment promote both the extrinsic and intrinsic apoptosis pathway in the BCSCs.
Resistance to TAM is the underlying cause of treatment failure that is frequently observed in breast cancer patients receiving TAM [74].Therefore, developing new therapeutic strategies to improve the clinical efficacy of Tamoxifen in breast cancer treatments is necessary. Combined treatment of chemotherapeutics with natural drugs has been reported to enhance the overall efficacy of chemotherapy treatments and minimize adverse side effects [75]. Interesting, we observed that pentadecanoic acid can improve the chemosensitivity of MCF-7/SC cells to TAM (Figure 7a and 7b). In addition, the results of FACs analysis indicated an accumulation in the sub-G1 populations as well as an accumulation of apoptosis population in MCF-7/SC cells following by combined treatment containing 100 μM of pentadecanoic acid and 10 μM of TAM (Figure 7d and 7e). Moreover,
results of western blot experiments confirmed that combined treatment of TAM together with pentadecanoic acid can increase the expression of apoptosis markers such as cleave-caspase 7, cleave-cleave-caspase 9 and cleave-PARP in MCF-7/SC cells (Figure 7e), indicating the potential use of petadecanoic acid/TAM combined therapy for breast cancer patients.
5. CONCLUSIONS
In summary, our study showed that pentadecanoic acid has more specific cytotoxicity in MCF-7/SC than non-stem cell MCF-7 and non-tumorigenic breast epithelial MCF-10A cells. Further, examination exhibited the mechanism that pentadecanoic acid can repress cell proliferation, stem cell properties as well as mobility capacity of the cell by suppressing JAK2/STAT3 signaling. Pentadecanoic acid also accumulated cell cycle arrest and promoted apoptosis in MCF-7/SC cells. This investigation provides the first evidence that pentadecanoic acid has the potential as a novel JAK2/STAT3 inhibitor to target breast cancer cells. Interestingly, pentadecanoic acid enhanced the chemosensitivity of MCF-7/SC cells to tamoxifen, suggesting that pentadecanoic acid has a prominent role in overcoming chemo-resistance, a major clinical challenge, in breast cancer.
REFERENCES
1. Siegel, R.L., K.D. Miller, and A. Jemal, Cancer statistics, 2016. CA: a cancer journal for clinicians, 2016. 66(1): p. 7-30.
2. Zielske, S.P., et al., Ablation of breast cancer stem cells with radiation. Translational oncology, 2011. 4(4): p. 227.
3. Fillmore, C.M. and C. Kuperwasser, Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast cancer research, 2008. 10(2): p. R25.
4. Collina, F., et al., Prognostic value of cancer stem cells markers in triple-negative breast cancer. BioMed research international, 2015. 2015.
5. Ginestier, C., et al., ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell, 2007. 1(5): p. 555-567.
6. Zinzi, L., et al., ABC transporters in CSCs membranes as a novel target for treating tumor relapse. Frontiers in pharmacology, 2014. 5: p. 163.
7. Yang, L., et al., Targeting cancer stem cell pathways for cancer therapy. Signal Transduction and Targeted Therapy, 2020. 5(1): p. 1-35.
8. Galoczova, M., P. Coates, and B. Vojtesek, STAT3, stem cells, cancer stem cells and p63. Cellular & molecular biology letters, 2018. 23(1): p. 12.
9. Berishaj, M., et al., Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Research, 2007. 9(3): p. R32.
10. McArthur, M.J., et al., Cellular uptake and intracellular trafficking of long chain fatty acids. Journal of lipid research, 1999. 40(8): p. 1371-1383.
11. Caballero, B., L.C. Trugo, and P.M. Finglas, Encyclopedia of food sciences and nutrition. 2003: Academic. 12. Pfeuffer, M. and A. Jaudszus, Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids.
Advances in Nutrition, 2016. 7(4): p. 730-734.
13. Jenkins, B., J.A. West, and A. Koulman, A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15: 0) and heptadecanoic acid (C17: 0) in health and disease. Molecules, 2015. 20(2): p. 2425-2444.
14. Adamska, A. and J. Rutkowska, Odd-and branched-chain fatty acids in milk fat--characteristic and health properties. Postepy higieny i medycyny doswiadczalnej (Online), 2014. 68: p. 998-1007.
15. Yang, Z., et al., Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer research, 2000. 60(3): p. 505-509. 16. Xu, C., et al., Heptadecanoic acid inhibits cell proliferation in PC‑9 non‑small‑cell lung cancer cells with acquired
gefitinib resistance. Oncology reports, 2019. 41(6): p. 3499-3507.
17. Nguyen, Y.T.-K., et al., Phenethyl Isothiocyanate Suppresses Stemness in the Chemo-and Radio-Resistant Triple-Negative Breast Cancer Cell Line MDA-MB-231/IR Via Downregulation of Metadherin. Cancers, 2020. 12(2): p. 268.
18. Menendez, J., et al., Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses her-2/neu (erb b-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (herceptin™) in breast cancer cells with her-2/neu oncogene amplification. Annals of oncology, 2005. 16(3): p. 359-371.
19. Jiang, L., et al., Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Scientific reports, 2017. 7(1): p. 1-11.
20. Moon, H.-S., S. Batirel, and C.S. Mantzoros, Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism, 2014. 63(11): p. 1447-1454.
21. Lu, X., et al., Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids in Health and Disease, 2010. 9(1): p. 106.
22. Rahaman, S.O., et al., Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene, 2002. 21(55): p. 8404-8413.
23. Marotta, L.L., et al., The JAK2/STAT3 signaling pathway is required for growth of CD44+ CD24–stem cell–like breast cancer cells in human tumors. The Journal of clinical investigation, 2011. 121(7): p. 2723-2735. 24. Puthier, D., R. Bataille, and M. Amiot, IL‑6 up‑regulates Mcl‑1 in human myeloma cells through JAK/STAT
rather than Ras/MAP kinase pathway. European journal of immunology, 1999. 29(12): p. 3945-3950. 25. Ecker, A., et al., The dark and the bright side of Stat3: proto-oncogene and tumor-suppressor. Front Biosci, 2009.
14(1): p. 2944-2958.
26. Chang, R., et al., Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nature communications, 2018. 9(1): p. 1-12.
27. Liu, J., et al., GGNBP2 suppresses triple-negative breast cancer aggressiveness through inhibition of IL-6/STAT3 signaling activation. Breast cancer research and treatment, 2019. 174(1): p. 65-78.
28. Pfeffer, C.M. and A.T. Singh, Apoptosis: a target for anticancer therapy. International journal of molecular sciences, 2018. 19(2): p. 448.
29. Christodoulou, M.-I., et al., Nature promises new anticancer agents: Interplay with the apoptosis-related BCL2 gene family. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 2014. 14(3): p. 375-399.
30. McLachlan, A., et al., Pancratistatin: a natural anti-cancer compound that targets mitochondria specifically in cancer cells to induce apoptosis. Apoptosis, 2005. 10(3): p. 619-630.
31. von Schwarzenberg, K. and A.M. Vollmar, Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer letters, 2013. 332(2): p. 295-303.
32. Kanai, M., et al., Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity involved in gastric cancer cell proliferation via MEK-ERK-dependent pathway. Oncogene, 2003. 22(4): p. 548-554.
33. Pancotti, F., et al., Caveolin-1 silencing arrests the proliferation of metastatic lung cancer cells through the inhibition of STAT3 signaling. Cellular signalling, 2012. 24(7): p. 1390-1397.
34. Green, D.R., Apoptotic pathways: the roads to ruin. Cell, 1998. 94(6): p. 695-698.
35. Ayob, A.Z. and T.S. Ramasamy, Cancer stem cells as key drivers of tumour progression. Journal of biomedical science, 2018. 25(1): p. 20.
36. Hardy, S., Y. Langelier, and M. Prentki, Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects1. Cancer research, 2000. 60(22): p. 6353-6358.
37. Listenberger, L.L., D.S. Ory, and J.E. Schaffer, Palmitate-induced apoptosis can occur through a ceramide-independent pathway. Journal of Biological Chemistry, 2001. 276(18): p. 14890-14895.
38. Mu, Y.-M., et al., Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology, 2001. 142(8): p. 3590-3597.
39. Zhang, Y., et al., Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids in health and disease, 2012. 11(1): p. 1.
40. Evans, L.M., et al., Stearate preferentially induces apoptosis in human breast cancer cells. Nutrition and cancer, 2009. 61(5): p. 746-753.
41. Hardy, S., et al., Saturated fatty acid-induced apoptosis in mda-mb-231 breast cancer cells a role for cardiolipin. Journal of Biological Chemistry, 2003. 278(34): p. 31861-31870.
42. De Angelis, M.L., F. Francescangeli, and A. Zeuner, Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers, 2019. 11(10): p. 1569.
43. Wang, L., et al., The role of CD44 and cancer stem cells, in Cancer Stem Cells. 2018, Springer. p. 31-42. 44. Nieto, M.A., Epithelial plasticity: a common theme in embryonic and cancer cells. Science, 2013. 342(6159): p.
1234850.
45. Nieto, M.A., et al., EMT: 2016. Cell, 2016. 166(1): p. 21-45.
46. Varga, J. and F.R. Greten, Cell plasticity in epithelial homeostasis and tumorigenesis. Nature cell biology, 2017. 19(10): p. 1133-1141.
47. Mani, S.A., et al., The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 2008. 133(4): p. 704-715.
48. Liu, S., et al., Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem cell reports, 2014. 2(1): p. 78-91.
49. Gilles, C., et al., Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition, in Rise and Fall of Epithelial Phenotype. 2005, Springer. p. 297-315.
50. Egeblad, M. and Z. Werb, New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2002. 2(3): p. 161-174.
51. Sternlicht, M.D. and Z. Werb, How matrix metalloproteinases regulate cell behavior. Annual review of cell and developmental biology, 2001. 17(1): p. 463-516.
52. Wang, B., et al., Expression and significance of MMP2 and HIF‑1α in hepatocellular carcinoma. Oncology letters, 2014. 8(2): p. 539-546.
53. Heissig, B., et al., Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell, 2002. 109(5): p. 625-637.
54. Ganesan, R., E. Mallets, and J. Gomez-Cambronero, The transcription factors Slug (SNAI2) and Snail (SNAI1) regulate phospholipase D (PLD) promoter in opposite ways towards cancer cell invasion. Molecular oncology, 2016. 10(5): p. 663-676.
55. Banerjee, K. and H. Resat, Constitutive activation of STAT 3 in breast cancer cells: A review. International journal of cancer, 2016. 138(11): p. 2570-2578.
56. Song, H., et al., A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proceedings of the National Academy of Sciences, 2005. 102(13): p. 4700-4705.
57. Kortylewski, M., R. Jove, and H. Yu, Targeting STAT3 affects melanoma on multiple fronts. Cancer and Metastasis Reviews, 2005. 24(2): p. 315-327.
58. Wendt, M.K., et al., STAT3 and epithelial–mesenchymal transitions in carcinomas. Jak-stat, 2014. 3(2): p. e28975.
59. Song, Y., et al., Fra-1 and Stat3 synergistically regulate activation of human MMP-9 gene. Molecular immunology, 2008. 45(1): p. 137-143.
60. Yue, P. and J. Turkson, Targeting STAT3 in cancer: how successful are we? Expert opinion on investigational drugs, 2009. 18(1): p. 45-56.
61. Siveen, K.S., et al., Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochimica et Biophysica Acta (BBA)-reviews on cancer, 2014. 1845(2): p. 136-154.
62. Lin, L., et al., STAT3 is necessary for proliferation and survival in colon cancer–initiating cells. Cancer research, 2011. 71(23): p. 7226-7237.
63. Corvinus, F.M., et al., Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia (New York, NY), 2005. 7(6): p. 545.
64. Xie, T.-x., et al., Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene, 2004. 23(20): p. 3550-3560.
65. Yang, J., et al., Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer research, 2005. 65(3): p. 939-947.
66. Yang, J. and G.R. Stark, Roles of unphosphorylated STATs in signaling. Cell research, 2008. 18(4): p. 443-451.
67. Zegeye, M.M., et al., Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Communication and Signaling, 2018. 16(1): p. 1-10.
68. Hirano, T., K. Ishihara, and M. Hibi, Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene, 2000. 19(21): p. 2548-2556.
69. Xie, Q., et al., Ilamycin C induces apoptosis and inhibits migration and invasion in triple-negative breast cancer by suppressing IL-6/STAT3 pathway. Journal of hematology & oncology, 2019. 12(1): p. 60.
70. McIlwain, D.R., T. Berger, and T.W. Mak, Caspase functions in cell death and disease. Cold Spring Harbor perspectives in biology, 2013. 5(4): p. a008656.
71. Parrish, A.B., C.D. Freel, and S. Kornbluth, Cellular mechanisms controlling caspase activation and function. Cold Spring Harbor perspectives in biology, 2013. 5(6): p. a008672.
72. Wolf, B.B. and D.R. Green, Suicidal tendencies: apoptotic cell death by caspase family proteinases. Journal of Biological Chemistry, 1999. 274(29): p. 20049-20052.
73. Chaitanya, G.V., J.S. Alexander, and P.P. Babu, PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Communication and Signaling, 2010. 8(1): p. 31.
74. Ali, S., et al., Molecular mechanisms and mode of tamoxifen resistance in breast cancer. Bioinformation, 2016. 12(3): p. 135.
75. Moon, J.Y., et al., Nobiletin enhances chemosensitivity to adriamycin through modulation of the Akt/GSK3β/β– catenin/MYCN/MRP1 signaling pathway in A549 human non-small-cell lung cancer cells. Nutrients, 2018. 10(12): p. 1829.